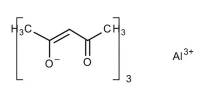
Nuclear reactions have the potential to make alchemists’ dreams of transforming one metal into another a reality, but at a high cost. Scientists believe they’ve discovered a way to achieve something almost as excellent — making a cheap metal act like a more costly metal – for a lot less money. It will alter energy storage and chemical manufacture if they are correct. Catalysts accelerate chemical processes by millions of times without being consumed themselves. Unfortunately, the most effective catalysts are extremely scarce and valuable for the most crucial processes for which they are required.
We may utilize either extremely expensive platinum or inexpensive nickel-iron alloys to create hydrogen from water, for example. Other processes, such as the breakdown of pollution from automobile engines, need even more esoteric metals such as rhodium and palladium. A team from the University of Minnesota discloses tailoring an ultrathin layer of alumina atop graphene to behave like other metals in the Journal of the American Chemical Society Au.
In a release, research author Dr. Tzia Ming Onn noted, “The considerable capacity to modify the catalytic and electrical characteristics of the catalyst surpassed our expectations.” The efficiency of catalysts is determined by the placements of their electrons and “holes,” which are defined by the lack of electrons. Catalysts’ surface layer, where they interact with reactive chemicals, is all that counts by nature. As a result, researchers created a 4 nanometer thick active alumina surface layer that can mimic the locations of atoms and holes in potent catalysts. Before an appropriate voltage was applied, this was deposited on top of graphene, which in turn rests atop an insulating internal layer. The team calls its device a “catalytic condenser.”
The study is based on semiconductor design and the use of ultrathin zinc-oxide layers to regulate the rate of electron transmission to solutions, according to the scientists. “The use of capacitive charging to boost thermocatalytic activity has not been documented to our knowledge,” the scientists write. Although the process catalyzed in this case – dehydrating the alcohol isopropanol – isn’t revolutionary, the team sees it as a proof of concept for far greater things.
Professor Dan Frisbie, one of the study’s authors, described the catalytic condenser as a “platform technology” that may be used in a variety of manufacturing applications. “We can modify the fundamental design ideas and innovative components to practically any chemical we can think of.” It’s a bold assertion, but if it holds true, it will significantly reduce the cost of processes that presently rely on costly catalysts. There will be significant environmental advantages as well: to obtain minuscule pieces of valuable metals, we must scrape large quantities of ore from the Earth, frequently in vulnerable areas.
All of this might be avoided by replacing them with a thin coating of plentiful alumina and a carbon-based product. Most notably, hydrogen and ammonia are two of the most promising possibilities for storing energy from intermittent sources, but they require better (or at least less expensive) catalysts to produce. The authors of the article are working on strategies to create more ecologically friendly polymers and eliminate the most harmful chemicals from waste gasses.