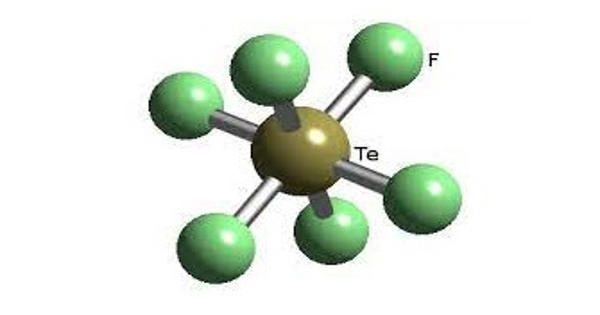
Tellurium hexafluoride is a colorless gas with a repulsive odor. It is the inorganic compound of tellurium and fluorine with the chemical formula TeF6. It is a byproduct of ore refining. It is a colorless, highly toxic gas with an unpleasant odor. It decomposes slowly in water to form hydrogen fluoride and tellurium ion. It is very toxic by inhalation and skin absorption.
Preparation
Tellurium hexafluoride can be prepared by treating tellurium with fluorine gas at 150 °C. It is prepared by the direct fluorination of tellurium metal. When heated to high temperatures, it may emit toxic fluoride and tellurium fumes. It can also be prepared by fluorination of TeO3 with bromine trifluoride. It is a by-product of ore refining. Upon heating, TeF4 disproportionates to give TeF6 and Te. It is heavier than air.
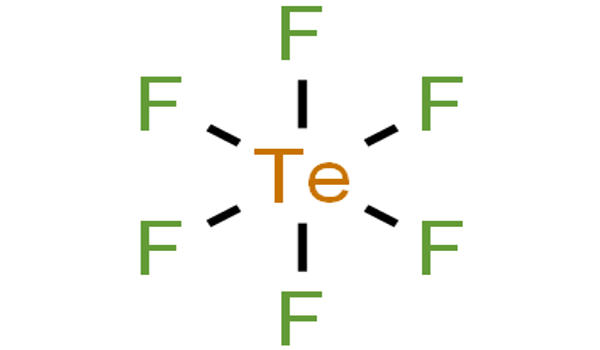
Properties
Tellurium hexafluoride is a highly symmetric octahedral molecule. Its physical properties resemble those of sulfur and selenium. It is less volatile, however, due to the increase in polarizability. At temperatures below −38°C, tellurium hexafluoride condenses to a volatile white solid.
- Formula mass: 241.59
- Melting point, °C: -37.6
- Boiling point: -38,9°C
- Sublimation point, °C: -38.9
- Vapor density (air=1): 8.3
- Critical temperature: 83
- Density: 4.006 g/cm3 (-191 C) (solid), 2.499 g/cm3 (-10 C) (liquid)
- Solubility in water: Reacts slowly
Reactivity
Tellurium hexafluoride is a tellurium coordination entity. Unlike SF6, tellurium hexafluoride is not chemically inert. For example, TeF6 slowly hydrolyzes to Te(OH)6:
TeF6 + 6 H2O → Te(OH)6 + 6 HF
Treatment of tellurium hexafluoride with tetramethylammonium fluoride (Me4NF) gives, sequentially, the hepta- and octafluorides:
TeF6 + Me4NF → Me4NTeF7
Me4NTeF7 + Me4NF → (Me4N)2TeF8
Toxicity
Tellurium hexafluoride is severely irritating and causes respiratory distress, pulmonary edema, and death in animals. In humans, it is reported to cause “garlic” breath, a metallic taste in the mouth, and fatigue. It is a respiratory irritant and humans may experience breathing difficulties after inhaling it. A small number of subjects occasionally reported loss of appetite and nausea. Inhalation of tellurium hexafluoride is expected to cause breathing difficulties in humans. Somnolence was observed only in the workers with the highest urinary concentrations of tellurium.
Information Source: