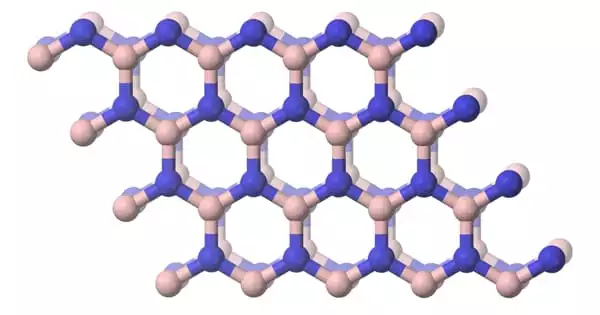
Boron nitride (BN) is a chemical compound with an equal number of boron and nitrogen atoms that is isoelectronic and isostructural to carbon. It is a thermally and chemically resistant boron and nitrogen refractory compound with the chemical formula BN. It can be found in a variety of crystalline forms that are isoelectronic to a similarly structured carbon lattice. It is a limited but important industrial ceramic material used primarily in electrical insulators and cutting tools.
The hexagonal form corresponding to graphite is the most stable and soft of the BN polymorphs and is thus used as a lubricant and cosmetic additive. The cubic (zincblende aka sphalerite structure) variety analogous to diamond is known as c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form.
Boron nitride ceramics have traditionally been used as parts of high-temperature equipment due to their excellent thermal and chemical stability. Boron nitride has potential applications in nanotechnology. BN nanotubes with a structure similar to carbon nanotubes, i.e. graphene (or BN) sheets rolled on themselves, can be produced, but their properties are very different.
Properties
- Molecular Weight: 24.82
- Appearance: Black solid in various forms
- Melting Point: 2973 °C
- Boiling Point: N/A
- Density: 2.1 g/cm3 (h-BN); 3.45 g/cm3 (c-BN)
- Solubility in H2O: Insoluble
Boron nitride (BN) empirical formula is deceptive. BN differs significantly from other diatomic molecules such as carbon monoxide (CO) and hydrogen chloride (HCl). Rather, it shares many similarities with carbon, whose representation as the monatomic C is also deceptive.
The partly ionic structure of BN layers in h-BN reduces covalency and electrical conductivity while increasing interlayer interaction, resulting in higher h-BN hardness compared to graphite. The absence of color and the large band gap in hexagonal-BN also indicate a reduced electron-delocalization. Because of the very different bonding – strong covalent within the basal planes (planes where boron and nitrogen atoms are covalently bonded) and weak between them – most properties of h-BN are highly anisotropic.
Hardness, electrical and thermal conductivity, for example, are much higher within planes than perpendicular to them. The properties of c-BN and w-BN, on the other hand, are more homogeneous and isotropic.
Those materials are extremely hard, with bulk c-BN having a slightly lower hardness and w-BN having a harder hardness than diamond. Polycrystalline c-BN with grain sizes of 10 nm has been reported to have Vickers hardness comparable to or higher than diamond.
Boron nitride can be doped p-type with beryllium and n-type with boron, sulfur, silicon, or carbon and nitrogen co-doped. Both hexagonal and cubic BN are wide-gap semiconductors with band-gap energies in the ultraviolet region. When h-BN or c-BN is charged, it emits UV light in the 215–250 nm range and can thus be used as light-emitting diodes (LEDs) or lasers.