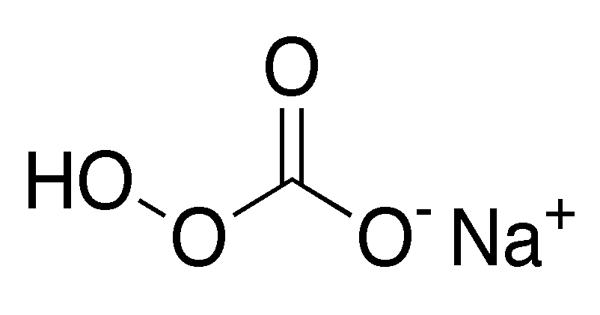
Sodium percarbonate, also called sodium carbonate peroxide, is an inorganic salt that typically appears as a colorless, crystalline solid. It is a chemical substance with the formula Na2H3CO6. It appears as a colorless, crystalline solid. It is an adduct of sodium carbonate (“soda ash” or “washing soda”) and hydrogen peroxide (that is, a perhydrate) whose formula is more properly written as 2 Na2CO3 · 3 H2O2.
It is denser than water. It is a colorless, crystalline, hygroscopic and water-soluble solid. It may combust in contact with organic materials. It is sometimes abbreviated as SPC. It contains 32.5% by weight of hydrogen peroxide. Its contact may irritate skin, eyes and mucous membranes. It may be toxic by ingestion. It is a bleaching and oxidizing agent commonly used in household cleaning products.
Sodium carbonate is a water-soluble mineral and ubiquitously present in the aqueous environment as there is equilibrium with carbon dioxide from the atmosphere and with other dissolved minerai carbonates. The product is used in some eco-friendly bleaches and other cleaning products, and as a laboratory source of anhydrous hydrogen peroxide. To enhance its stability, percarbonate is frequently marketed as granules coated with a protective disilicate-based layer.
Structure
At room temperature, solid sodium percarbonate has the orthorhombic crystal structure, with the Cmca crystallographic space group. The structure changes to Pbca as the crystals are cooled below about −30°C.

Production
Sodium percarbonate is produced industrially by crystallization of a solution of sodium carbonate and hydrogen peroxide, with proper control of the pH and concentrations. This is also a convenient laboratory method.
Alternatively, dry sodium carbonate may be treated directly with concentrated hydrogen peroxide solution.
It may also be formed from a process starting from sodium peroxide When absolute ethyl alcohol reacts with sodium peroxide at 0° C. a perhydroxide is produced.
C2H5OH + Na2O2 – O:NaOH + C2H2ONa.
Carbon dioxide converts it into sodium hydrogen percarbonate.
World production capacity of this compound was estimated at several hundred thousand tons for 2004.
Uses
- Sodium percarbonate helps whiten and clean during washing. Whole Foods has deemed the ingredient acceptable in its body care and cleaning product quality standards.
- As an oxidizing agent, sodium percarbonate is an ingredient in a number of home and laundry cleaning products, including non-chlorine bleach products such as Oxyper, OxiClean, Tide laundry detergent, and Vanish.
- Commercially, manufacturing sodium percarbonate involves reacting sodium carbonate with hydrogen peroxide and then crystallizing the mixture.
- Many commercial products mix a percentage of sodium percarbonate with sodium carbonate. The average percentage of an “Oxy” product in the supermarket is 65% sodium percarbonate and 35% sodium carbonate.
- Sodium percarbonate is also used as a cleaning agent by many home brewers. It is often used in toothpaste and tooth whitening products, as well as in crop production to inhibit algae and moss.
Information Source: