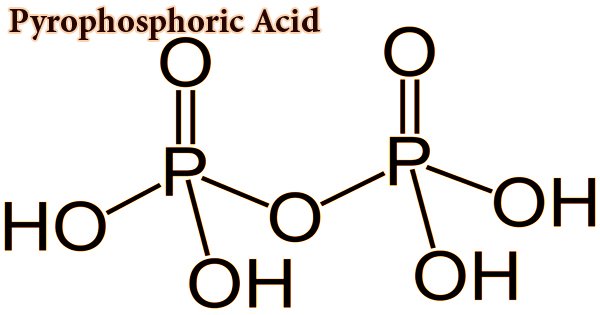
Pyrophosphoric acid, also known as diphosphoric acid, is by nature a medium-strong hygroscopic inorganic acid and is both a colorless and odorless chemical. With the formula H4P2O7 or, more descriptively, ((HO)2P(O))2O, the inorganic compound. Water, diethyl ether, and ethyl alcohol are soluble in it. By dehydration, it is made from phosphoric acid. Pyrophosphoric acid slowly hydrolyzes into phosphoric acid in the presence of water. Two polymorphs, which melt at 54.3 °C and 71.5 °C, crystallize the anhydrous acid. The compound is not especially useful, except that it is a component of polyphosphoric acid and the conjugate acid of the pyrophosphate anion. Pyrophosphates are called anions, salts, and esters of pyrophosphoric acid.
Pyrophosphoric acid may be prepared by heating orthophosphoric acid at 215°C:
2H3PO4 → H4P2O7 + H2O
3D model of Pyrophosphoric Acid
By ion exchange, passing an aqueous solution of sodium pyrophosphate, Na4P2O7, through an effective cation exchange column, the acid solution in pure form can be obtained. Pyrophosphoric Acid was invented in 1827 by Mr. Clarke of Glasgow. It is an anhydride of acyclic phosphorus acid that is obtained by condensation of two phosphoric acid molecules. By ion exchange from sodium pyrophosphate or by treating lead pyrophosphate with hydrogen sulfide, pyrophosphoric acid is best prepared. It is not prepared through the phosphoric acid dehydration method. Pyrophosphoric acid is, instead, generated as just one of the ingredients.
There are four replaceable H+ ions in the acid. Its constants of dissociation show that two H+ ions are strongly acidic while the other two protons are weakly acidic. In particular, the first dissociation constant is very high:
H4P2O7 + H2O ↔ H3O+ + H3P2O7¯ Ka1 ~ 10–1
H3P2O7¯+ H2O ↔ H3O+ + H2P2O72– Ka2 ~ 1.5×10–2
PHOSPHORIC ACID, PYRO 701H2P2O72¯ + H2O ↔ H3O+ + HP2O73– Ka3 ~ 2.7×10–7
HP2O73¯ + H2O ↔ H3O+ + P2O74¯ Ka4 ~ 2.4×10–10
Pyrophosphoric acid is formed in an aqueous solution, like all polyphosphoric acids, hydrolyses, and finally a balance between phosphoric acid, pyrophosphoric acid, and polyphosphoric acids. Crystallizes in two anhydrous forms: a metastable form melting at 54.3 °C and a second and more solid form melting at 71.5 °C; highly soluble in cold water, quite slowly reacting to form phosphoric acid; much faster decomposition in hot water; very soluble in alcohol and ether. Pyrophosphoric acid is an inorganic acid which is moderately solid. The pyro-acid is produced as low as 100 °C, according to early researchers, and the conversion between 255 and 260 °C is complete.
Information Sources: