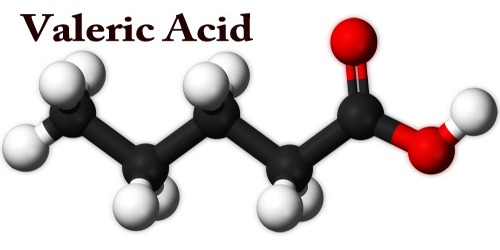
Valeric acid, or pentanoic acid, is a colorless, oily liquid with an unpleasant odor. It is highly corrosive and must be handled with care. It is a straight-chain alkyl carboxylic acid with the chemical formula CH3(CH2)3COOH. Like other low-molecular-weight carboxylic acids, it has an unpleasant odor.
Valeric acid is found naturally in the perennial flowering plant valerian (Valeriana officinalis), from which it gets its name. Its primary use is in the synthesis of its esters. Volatile esters of valeric acid tend to have pleasant odors and are used in perfumes and cosmetics. Ethyl valerate and pentyl valerate are used as food additives because of their fruity flavors.
Valeric acid is mainly used as a chemical intermediate to manufacture flavors and perfumes, synthetic lubricants, agricultural chemicals, and pharmaceuticals. It is also used as a flavoring aid in foods. Valeric acid is considered as safe as a food additive by the World Health Organization (WHO).
Valeric acid is a straight-chain saturated fatty acid containing five carbon atoms. It has a role as a plant metabolite. It is a short-chain fatty acid and a straight-chain saturated fatty acid. It is the conjugate acid of a valerate.
Valeric acid is one volatile component in swine manure. Other components include other carboxylic acids, skatole, trimethylamine, and isovaleric acid. It can cause irritation if it comes into contact with the skin, eyes, or mucous membranes.
Valeric acid is a carboxylic acid. Exothermically neutralizes bases, both organic and inorganic, producing water and a salt. Can react with active metals to form gaseous hydrogen and a metal salt. Reacts with cyanide salts to generate gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by reaction with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Reacts with sulfites, nitrites, thiosulfates, and dithionites to generate flammable and/or toxic gases and heat. Reacts with carbonates and bicarbonates to generate a harmless gas (carbon dioxide) but still heat. Can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. May initiate polymerization reactions. May catalyze (increase the rate of) chemical reactions.
Valeric acid appears as a colorless liquid with a penetrating unpleasant odor. Density 0.94 g / cm3. Freezing point -93.2°F (-34°C). Boiling point 365.7°F (185.4°C). Flashpoint 192°F (88.9° C). Corrosive to metals and tissue.
Information Sources: