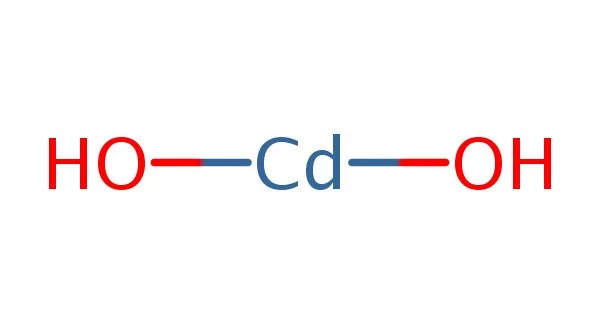
Cadmium hydroxide, also known as Cd(OH)2, is an inorganic compound. It is a white crystalline ionic compound used in nickel-cadmium batteries. It is employed in the fabrication of negative electrodes for cadmium-nickel batteries. It is useful in biochemical research for proteomics. It is suitable for cadmium plating and cadmium salt.
Properties
It is a white crystalline ionic compound that is a key component of NiCd batteries. The hydroxide is used in place of cadmium oxide for variety of operations. It is a white powder or crystal; trigonal or hexagonal crystal system; density 4.79 g/cm3; decomposes slowly at 130°C; dehydration completes at 300°C; insoluble in water (2.6 mg/L at 20°C); soluble in dilute acids.
- Chemical formula: Cd(OH)2
- Molar mass: 146.43 g/mol
- Appearance: white crystals
- Density: 4.79 g/cm3
- Melting point: 130 °C (266 °F; 403 K)
- Boiling point: 300 °C (572 °F; 573 K) (decomposes)
- Solubility in water: 0.026 g/100 mL
- Solubility product (Ksp): 7.2×10−15
- Solubility: soluble in dilute acids
- Magnetic susceptibility (χ): -41.0·10−6 cm3/mol
- Crystal structure: hexagonal

Preparation and reactions
Cadmium hydroxide adopts the same structure as Mg(OH)2, consisting of slabs of octahedral metal centers surrounded by octahedral of hydroxide ligands.
It is produced by treating cadmium nitrate with sodium hydroxide:
Cd(NO3)2 + 2 NaOH → Cd(OH)2 + 2 NaNO3
Cadmium hydroxide may be precipitated by adding any cadmium salt solution to a boiling solution of caustic soda or caustic potash:
CdCl2 + 2NaOH → Cd(OH)2 + 2NaCl.
Preparation has been achieved from some other cadmium salts,
Cd(OH)2 and cadmium oxide react equivalently. Cadmium hydroxide is more basic than zinc hydroxide. It forms the anionic complex [[Cd(OH)4]]2− when treated with concentrated base. It forms complexes with cyanide, thiocyanate, and ammonia.
When cadmium hydroxide is heated, it loses water and produces cadmium oxide. Decomposition begins at 130 °C and ends at 300 °C. The corresponding cadmium salts are produced by reactions with mineral acids (HX) (CdX2). Cadmium chloride, cadmium sulfate, and cadmium nitrate are the products of hydrochloric acid, sulfuric acid, and nitric acid, respectively.
Uses
It is generated in storage battery anodes, in nickel-cadmium and silver-cadmium storage batteries in its discharge:
2 NiO(OH) + 2 H2O + Cd → Cd(OH)2 + Ni(OH)2