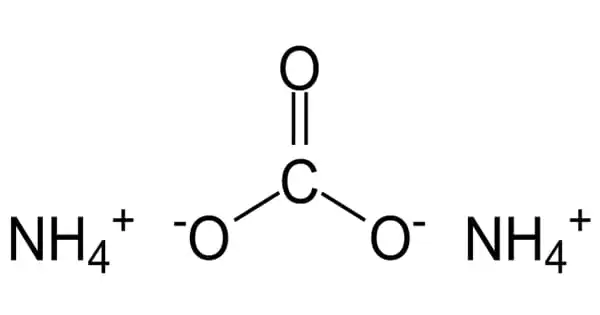
Ammonium carbonate is a salt having the chemical formula (NH4)2CO3. It’s a chemical compound made up of ammonium and carbonate ions. Because it degrades quickly to gaseous ammonia and carbon dioxide when heated, it is employed as a leavening agent as well as a smelling salt. Because it degrades to gaseous ammonia and carbon dioxide when heated, it is commonly used as a leavening agent and smelling salt. It is extremely detrimental to the environment, and serious steps should be taken to avert it quickly.
It was a precursor to the more contemporary leavening ingredients baking soda and baking powder and is also known as baker’s ammonia. It is a component of what was previously known as sal volatile and salt of hartshorn, and when cooked, it emits a pungent odor.
Properties
It is a colorless crystalline solid or white powder that has a strong ammonia odor and a sharp ammoniacal taste. It is non-combustible and water-soluble. It produces ammonium salt and carbon dioxide when it combines with acids. It creates ammonia gas when it interacts with bases. It’s made by combining carbon dioxide and aqueous ammonia.
- Chemical formula: (NH4)2CO3
- Molar mass: 96.09 g/mol
- Appearance: White powder
- Density: 1.50 g/cm3
- Melting point: 58 °C (136 °F; 331 K) (decomposes)
- Boiling point: Decomposes

Production
Ammonium carbonate is created by combining carbon dioxide and aqueous ammonia. However, as of 1997, approximately 80000 tons per year were produced. Carbon dioxide can be used to make ammonium carbonate. This entails injecting 99-99.99 percent pure carbon dioxide and ammonia gas into two buffer gas vats at pressures ranging from 0.2 to 2MPa.
Carbon dioxide and aqueous ammonia are combined to make ammonium carbonate. As of 1997, the annual output was over 80,000 tons. It is possible to have an orthorhombic monohydrate. In an ammonia solution exposed to a carbon dioxide-rich atmosphere, it crystallizes.
Decomposition
Ammonium carbonate slowly decomposes at standard temperature and pressure through two pathways. Thus any initially pure sample of ammonium carbonate will soon become a mixture including various byproducts.
Ammonium carbonate can spontaneously decompose into ammonium bicarbonate and ammonia:
(NH4)2CO3 → NH4HCO3 + NH3
Which further decompose to carbon dioxide, water and another molecule of ammonia:
NH4HCO3 → H2O + CO2 + NH3
Uses
Ammonium carbonate can be used as a leavener in traditional recipes, notably those from Northern Europe and Scandinavia (for example, Speculoos, Tunnbröd, or Lebkuchen). It was the forerunner of today’s more widely used baking powder.
Originally manufactured from pulverized deer horn and known as hartshorn, it is now known as baker’s ammonia. It is made by sublimating a mixture of ammonium sulfate and calcium carbonate, and it comes in the form of a white powder or a hard, white, or translucent mass. It serves as a heat-activated leavening agent, converting to carbon dioxide (leavening), ammonia (which must be dissipated), and water. It is occasionally used with sodium bicarbonate to act as a double-acting baking powder and to help cover any ammonia odor that has not been baked out.