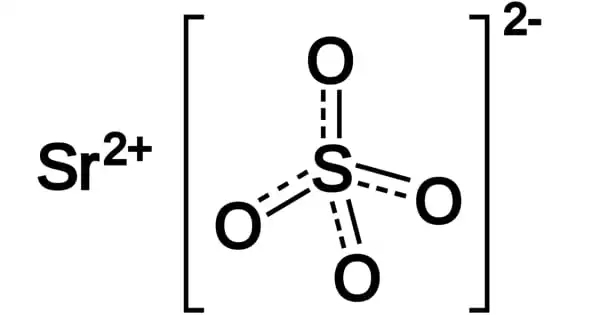
Strontium sulfate (SrSO4) is the strontium sulfate salt. In crystalline form, it is white in color. It is found in nature as the mineral celestine and is a white crystalline powder. It dissolves in water at a rate of 1 part in 8,800. It is significantly soluble in alkali chloride solutions and more soluble in dilute HCl and nitric acid (e.g. sodium chloride). Celestine – the mineral – occurs naturally in the environment.
In the early nineteenth century, strontium sulfate was occasionally used as a white artist pigment in England. It was quickly supplanted by barium sulfate. Strontium sulfate is currently used as a red colorant in pyrotechnics, ceramics, and glass.
Structure
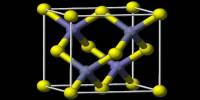
Strontium sulfate is a polymeric material that is structurally equivalent to barium sulfate. Acantharea, a small group of radiolarian protozoa, uses crystallized strontium sulfate as a major constituent of their skeleton. It is insoluble in water but soluble in nitric acid, dilute HCl, and alkali chloride solutions.
Properties
White crystalline powder; orthorhombic crystals; refractive index 1.622; hardness 3.3 Mohs; density 3.96 g/cm3; melts at 1,605°C; very slightly soluble in water 0.014 g/100mL at 30°C; soluble in alkali chloride solutions; slightly soluble in alcohol; insoluble in alkalis.
- Molecular Weight: 183.63
- Appearance: White crystals or crystalline powder
- Melting Point: 1,606 °C
- Boiling Point: N/A
- Density: 3.96 g/cm3
- Solubility in H2O: 125 mg/L (25 °C)
- pH: 7.9 (10 g/L)
- Exact Mass: 183.857 g/mol

Production
Strontium sulfate occurring in nature as celestite is mined from its natural deposits. Also, the sulfate can be made by reacting strontium oxide, hydroxide, or carbonate with dilute hydrochloric acid:
SrO + H2SO4 → SrSO4 + H2O
SrCO3 + H2SO4 → SrSO4 + CO2 + H2O
Strontium sulfate has the molecular formula of SrSO4 and a molecular weight of 183.6842 g/mol. It can be prepared by the reaction of sodium sulfate and strontium chloride:
Na2SO4 + SrCl2 → SrSO4 + 2NaCl
Its hardness is 3.3 Mohs. It melts at 1608°C. Its solubility in water is 0.015 g/100 ml at 25°C and 0.014 g at 35°C. It is insoluble in ethanol and acetone, but slightly soluble in acids and alkalis. It is soluble in alkali chloride solutions. The heat of formation, △Hf, is –1453 kJ/mol with an entropy value, S0, of 117.0 J/mol K.
The pH of a water solution is 7.5. It has a specific gravity of 3.8 to 4.0 g/cm3. Pure strontium sulfate precipitate is made up of single crystals a few tens of microns long with distinct growth steps. Crystals grown with polyvinyl sulfonate in stirred solutions exhibit a striking change of habit: they are sponge-like structures composed of nanometer-sized crystallites. A small group of protozoa uses crystallized strontium sulfate as a major component of their skeleton. It is occasionally used to add iridescence to glass and pottery glazes, as well as as a fining agent in crystal glass.
Applications and chemistry
Strontium sulfate is of interest as a naturally occurring precursor to more useful strontium compounds. In industry, it is converted to carbonate, which is used as a ceramic precursor, and nitrate, which is used in pyrotechnics.
Strontium sulfate’s low aqueous solubility can cause scale formation in processes where these ions interact. Depending on the groundwater conditions, it can form on the surfaces of equipment in underground oil wells, for example.
Occurrences
Strontium sulfate occurs naturally as the mineral celestite, which is the main strontium ore. The sulfate is the raw material used to make strontium metal and nearly all of its salts. Sulfate is also used in pyrotechnics and ceramics.