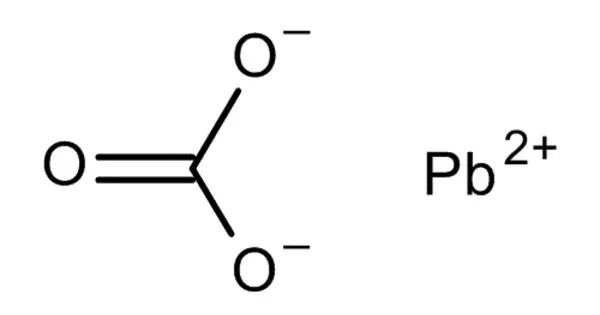
The chemical compound PbCO3 is lead(II) carbonate. Despite its toxicity, it is a white solid with a number of practical applications. It has many applications. It catalyzes formaldehyde polymerization into high molecular weight crystalline poly(oxymethylene) products. It’s used in poly(vinyl chloride) friction liners for pulleys on hoisting engine drive cables.
In nature, lead carbonate occurs as the mineral cerussite. It has several uses. The compound is used in high pressure lubricating greases, as a coating on polyvinyl chloride to improve the dielectric properties of the polymers, in PVC friction liners for pulleys, in corrosion-resistant grids in lead-storage batteries, in heat-sensitive sheets for thermographic copying, as an electrophotography photoconductor, in thermistors, and in waxes for steel cables.
Properties
Colorless orthorhombic crystals; refractive index 1.804; Moh’s hardness 3–3.5; density 6.60 g/cm3; decomposes on heating at 315°C; practically insoluble in water (1.1 mg/L at 20°C); KSP 1.46×10–13 at 25°C; also insoluble in alcohol and ammonia; soluble in acids and alkalies.
- Chemical formula: PbCO3
- Molar mass: 267.21 g/mol
- Appearance: White powder
- Density: 6.582 g/cm3
- Melting point: 315 °C (599 °F; 588 K) (decomposes)
- Solubility in water: 0.00011 g/100 mL (20 °C)
- Solubility product (Ksp): 1.46 x 10−13
- Solubility: insoluble in alcohol, ammonia; soluble in acid, alkali

Structure
Lead(II) carbonate, like all metal carbonates, has a dense, highly crosslinked structure composed of intact CO32- and metal cation sites. The Pb(II) centers are seven-coordinate, as confirmed by X-ray crystallography, and are surrounded by multiple carbonate ligands. The carbonate centers are bonded to a single Pb and bridge to five additional Pb sites.
Preparation
Lead carbonate is prepared by passing carbon dioxide into a cold dilute solution of lead acetate:
Pb(C2H3O2)2 + CO2 + H2O → PbCO3 + CH3COOH
The compound also is prepared in the laboratory by adding sodium bicarbonate to a cold dilute solution of a lead(II) salt, such as lead nitrate or acetate:
Pb2+ + 2HCO3– → PbCO3 + CO2 + H2O
Production and use
Lead carbonate is manufactured by passing carbon dioxide into a cold dilute solution of lead(II) acetate, or by shaking a suspension of a lead salt more soluble than the carbonate with ammonium carbonate at a low temperature to avoid formation of basic lead carbonate.
Pb(CH3COO)2 + (NH4)2CO3 → PbCO3 + 2 NH4(CH3COO)
Lead carbonate is used as a catalyst to polymerize formaldehyde to poly(oxymethylene). It improves the bonding of chloroprene to wire.
Regulations
This compound’s supply and use are restricted in Europe. Another important application of this compound is in catalysis, where it is used to catalyze the polymerization of formaldehyde to high molecular weight polymeric products and to speed up the curing process of moldable thermosetting silicone resins.
Toxicity
Despite being an insoluble salt of lead, ingestion of the compound has low-to-moderate systemic effects in humans. The symptoms include gastrointestinal contractions, jaundice, convulsions, nausea or vomiting, and brain degeneration.