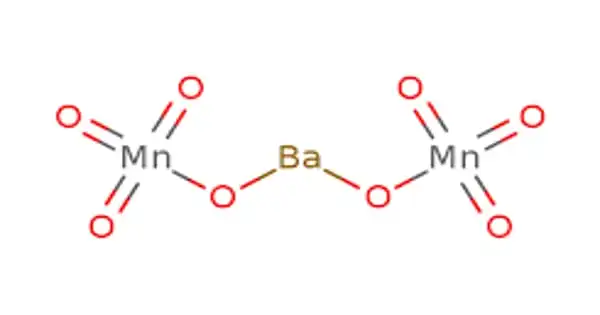
The chemical compound barium permanganate has the formula Ba(MnO4)2. It has the appearance of a purplish-colored crystalline solid. It is noncombustible, however, it speeds up the combustion of combustible materials. It crystallizes into violet to brown crystals that are only slightly soluble in water. When it comes into contact with combustible liquids, it may spontaneously ignite. It is employed as a disinfectant and in the production of various permanganates.
Properties
Barium permanganate is a useful oxidation reagent that is simple to manufacture. This reagent converts many types of primary and secondary hydroxy compounds to their carbonyl derivatives. It is a purplish-colored crystalline solid that is employed as a disinfectant and in the production of other permanganates.
- Molecular Weight: 375.198
- Appearance: Dark purple crystals
- Melting Point: 200 °C (dec.)
- Boiling Point: N/A
- Density: 3.77 g/cm3
- Solubility in H2O: 6.25 g/100 mL (29 °C)

Preparation
Barium permanganate may be produced by disproportionation of barium manganate in a mildly acidic solution, including solutions carbon dioxide or sulfuric acid:
3 BaMnO4 + 2 CO2 → Ba(MnO4)2 + 2 BaCO3 + MnO2
3 BaMnO4 + 2 H2SO4 → Ba(MnO4)2 + 2 BaSO4 + MnO2 + 2 H2O
It is also possible to make it by oxidizing barium manganate using powerful oxidants. Preparations based on aqueous reactions of barium manganate are extremely sluggish due to the manganate’s low solubility.
Another method for producing barium permanganate is through the interaction of silver permanganate and barium chloride. Highly pure samples can be made using a similar reaction between potassium permanganate and aluminum sulfate, which results in aluminium permanganate, which is then reacted with a stoichiometric quantity of barium hydroxide.
Reactions
Barium permanganate is a strong oxidizer. It is thermally stable up to 180°C, above which it decomposes in two stages between 180–350 and 500–700°C.
2 Ba(MnO4)2 → 2 BaMnO3 + 2 MnO2 + 3 O2
4 BaMnO3 → 4 BaO + 2 Mn2O3 + O2
It has been demonstrated that breakdown occurs at slow rates above 160 °C and that irradiation with UV or X-rays reduces this temperature. The mechanism is influenced by crystal flaws and impurities.
Permanganic acid can be made by reacting dilute sulfuric acid with a solution of barium permanganate, with the insoluble barium sulfate result being filtered out:
Ba(MnO4)2 + H2SO4 → 2 HMnO4 + BaSO4
The sulfuric acid employed must be dilute; reactions of permanganates with strong sulfuric acid produce manganese heptoxide as anhydride.
Reactivity Profile
If acetic acid or acetic anhydride is not kept cool, it might explode when combined with permanganates such as Barium permanganate. Explosions can occur when sulfuric acid-treated permanganates come into contact with benzene, carbon disulfide, diethyl ether, ethyl alcohol, petroleum, or organic debris.
Applications
It is used to make dry cells and other permanganates, and as a disinfectant.