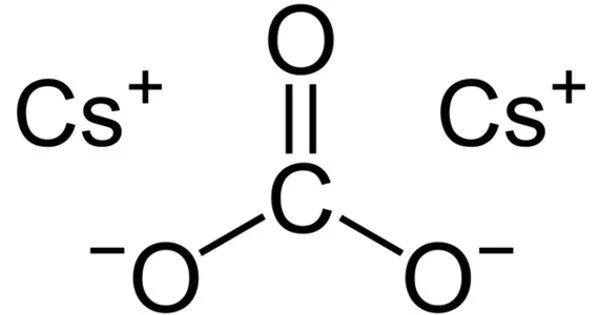
Cesium carbonate is a non-metallic compound. Under normal conditions of temperature and pressure, it is a white solid. It dissolves readily in polar solvents such as water, alcohol, and DMF. It is easily soluble in water and absorbs moisture quickly when exposed to air. Because cesium carbonate aqueous solution is highly alkaline, it can react with acid to produce corresponding cesium salts and water while emitting carbon dioxide. It is more soluble in organic solvents than other carbonates such as potassium and sodium carbonates, but it is insoluble in other organic solvents such as toluene, p-xylene, and chlorobenzene.
Cesium carbonate is a common cesium salt that is easy to transform and can be used as a precursor to other cesium salts. This compound is used in organic synthesis as a base. It also appears to have applications in energy conversion.
Properties
- Chemical formula: Cs2CO3
- Molar mass: 325.82 g/mol
- Appearance: white powder
- Density: 4.072 g/cm3
- Melting point: 610 °C (1,130 °F; 883 K) (decomposes)
- Solubility in water: 2605 g/L (15 °C)
- Solubility in ethanol: 110 g/L
- Solubility in dimethylformamide: 119.6 g/L
- Solubility in dimethyl sulfoxide: 361.7 g/L
- Solubility in sulfolane: 394.2 g/L
- Solubility in methylpyrrolidone: 723.3 g/L

Preparation
Caesium carbonate can be prepared by the thermal decomposition of caesium oxalate. Upon heating, caesium oxalate is converted to caesium carbonate with the emission of carbon monoxide.
Cs2C2O4 → Cs2CO3 + CO
It can also be synthesized by reacting caesium hydroxide with carbon dioxide.
2 CsOH + CO2 → Cs2CO3 + H2O
The method of preparing cesium carbonate using cesium alum as a raw material:
(1) To recrystallize cesium alum, dissolve it in deionized water and heat to dissolve; after dissolving, cool to crystallize and filter; and after filtering, obtain refined cesium alum.
(2) After dissolving refined cesium alum in deionized water, obtain refined cesium alum solution, slowly drip lime milk into refined cesium alum solution, and obtain cesium sulfate net solution after solid-liquid separation;
(3) Causticizing, the barium hydroxide solution is added to the cesium sulfate clean liquid 2 to 3 times, and the barium sulfate solid and causticizing liquid are separated by centrifugation.
(4) Carbonization, concentration, and evaporation add CO to the causticizing solution for carbonization, carbonize until the pH of the carbonization solution is 7 to 10, stand still, concentrate the carbonization supernatant after standing, and obtain a concentrated solution after cooling;
(5) After fine filtration and drying, the concentrated liquid is filtered with a polymer filter device, and the filtered filtrate is concentrated, crystallized, and dried at low temperature to obtain a low-temperature material.
(6) High-temperature decomposition; after high-temperature decomposition of low-temperature material, high-temperature material is obtained.
(7) Secondary fine filtration, the high-temperature material is dissolved in deionized water, and then filtered with a polymer filter device, and the filtered liquid is obtained after filtration;
(8) The clean liquid is concentrated and dried, and the filtered clean liquid is concentrated and dried to obtain cesium carbonate.
Chemical reactions
Caesium carbonate is very important for the N-alkylation of compounds such as sulfonamides, amines, β-lactams, indoles, heterocyclic compounds, N-substituted aromatic imides, phthalimides, and several similar other compounds.
Research on these compounds has focused on their synthesis and biological activity. In the presence of sodium tetrachloroaurate (NaAuCl4), caesium carbonate is very efficient mechanism for aerobic oxidation of different kinds of alcohols into ketones and aldehydes at room temperature without additional polymeric compounds. There is no acid formation produced when primary alcohols are used.
Uses
To improve the device’s conversion efficiency, cesium carbonate can be used as a modified layer between the active layer of the solar cell and the Al electrode.
It is a versatile organic synthesis reagent. This inorganic base has been used in a variety of organic applications, including N-protection of amino acids and as a base in the Horner-Wadsworth-Emmons reaction and Suzuki couplings. It has also been used as a catalyst for ethylene oxide polymerization, as a coating for spatter-free steel welding in CO2, as a functional interlayer in photovoltaic devices, and in oxide cathodes.