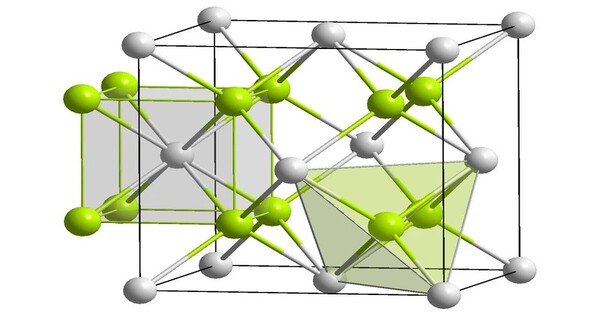
Radium fluoride is an inorganic compound with a chemical formula of RaF2. This salt, like all radium compounds, is highly radioactive. It is an inorganic salt and is part of the larger family of alkaline earth metal fluorides. It can be coprecipitated with lanthanide fluorides. It has the same crystal form as calcium fluoride (fluorite). However, calculations suggest that radium fluoride vapor consists of RaF2 molecules, with a bond angle of 118°, due to substantial covalent interaction within the molecule. Radium is a radioactive element, and as such, radium fluoride is radioactive as well.
Radium fluoride is highly radioactive due to the presence of radium. Radium itself is an alpha-emitting radioactive element, and its isotopes decay into radon gas. It is generally poorly soluble in water, similar to other alkaline earth metal fluorides. However, it may dissolve to some extent in acidic solutions. It typically forms a crystalline structure similar to calcium fluoride (CaF₂), with a cubic crystal system.
Production
Radium fluoride can be produced by the reaction of radium metal and hydrogen fluoride gas:
Ra + 2 HF → RaF2 + H2
Properties
- Chemical formula: RaF2
- Molar mass: 263.8214 g/mol
- Appearance: White cubic crystals
- Density: 6.7 g/cm3
Occurrences
In Nature: Radium fluoride is not typically found in nature in significant quantities. However, it may occur in small amounts as part of uranium ores, particularly in mineral deposits where radium is present, like pitchblende (now known as uraninite), which contains uranium and radium. In these ores, radium can react with fluorine to form radium fluoride under specific conditions.
Synthetic Production: It is typically synthesized in laboratories or industrial settings by reacting radium compounds, such as radium chloride (RaCl₂), with hydrofluoric acid (HF). This reaction produces radium fluoride and hydrogen chloride (HCl).
The process is as follows:
RaCl2+2HF→RaF2+2HCl
Applications
Due to its high radioactivity, radium fluoride doesn’t have many widespread applications today. However, it has historically been of interest in radiology and radioactivity studies. Some potential uses or areas of interest include:
- Radium Isotope Research: As part of experiments involving radioactive isotopes, radium fluoride might have been used in the past, though safer alternatives are now favored due to the hazards posed by radium.
- Radiotherapy: While radium itself has been used in radiotherapy for treating cancers (primarily in the form of radium salts like radium-226), radium fluoride is not commonly used in this context due to the associated risks and the development of more effective and safer radiotherapy methods.
Safety and Hazards
- Radioactivity: Radium fluoride is highly radioactive, emitting alpha particles, which are harmful if the substance is ingested, inhaled, or enters the body through a wound. Alpha particles can cause severe cellular damage when deposited inside tissues.
- Radiation Protection: Handling radium fluoride requires strict safety protocols to avoid radiation exposure. This includes the use of shielding (such as lead) and protective gear, as well as working with the compound in controlled environments, such as fume hoods.
- Decay Products: The decay of radium produces radon gas (a radioactive gas) which is a significant health hazard. Prolonged exposure to radon is a leading cause of lung cancer.