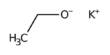
Phosphide carbides, also known as carbide phosphides, are compounds that include anions of carbide (C4−) and phosphide (P3−). They can be classified as mixed anionic chemicals. Related compounds are phosphide silicides, germanide phosphides, arsenide carbides, nitride carbides, and silicide carbides.
Phosphide carbides can be manufactured using a variety of processes, including solid-state reactions, chemical vapor deposition (CVD), and others that provide for compositional and structural control.
Structure
Because the carbon atoms inside the metal lattice are interstitial, phosphorus carbides frequently adopt complicated crystal forms. These structures have an impact on the physical and chemical properties of materials.
Light rare earth phosphide carbides contain the ethenide ion [C=C]4−. P and C2 are disordered in these, and they substitute for each other at random, notwithstanding the charge difference. Because the bonds between carbon and phosphorus are weak, these molecules lack a C-P bond and instead consist of carbon or phosphorus standing alone.
Composition
The exact composition can vary widely depending on the metal involved and the synthesis conditions. Common metals include transition metals like iron (Fe), nickel (Ni), cobalt (Co), and others. These can be made by heating a metal, red phosphorus and graphite powder together in a carbon crucible under an inert gas atmosphere.
Properties
They can exhibit a variety of characteristics based on their composition and structure. Some phosphide carbides are noted for their hardness and wear resistance, making them ideal for cutting tools and wear-resistant coatings. Others may have electrical or magnetic properties that make them useful in electronic applications.
Applications
Their uses include metallurgy, catalysis, and electronics. Because of its unusual electrical structure, tungsten phosphide carbide (WPC) has been investigated for use as a catalyst in hydrogen evolution reactions.