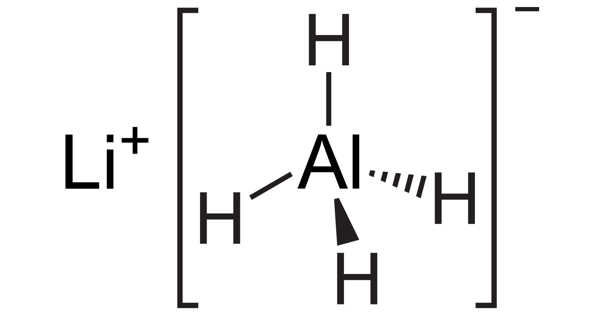
LAH, or lithium aluminum hydride, is an inorganic compound with the chemical formula LiAlH4. It’s a dark grey solid. Finholt, Bond, and Schlesinger discovered it in 1947. This compound is used in organic synthesis as a reducing agent, particularly for the reduction of esters, carboxylic acids, and amides. The solid is extremely reactive to water, emitting gaseous hydrogen (H2).
Properties
Lithium aluminum hydride is a white powder that turns gray when exposed to air. Friction can cause ignition if it is spread out over a large flat combustible surface. Although LAH is a colorless solid, commercial samples are typically gray due to contamination. Recrystallization from diethyl ether can be used to purify this material. A Soxhlet extractor is used in large-scale purifications. Because the impurities are innocuous and easily separated from the organic products, impure gray material is commonly used in synthesis.
The pure powdered material, but not the large crystals, is pyrophoric. Mineral oil is used in some commercial materials to inhibit reactions with atmospheric moisture, but it is more commonly packed in moisture-proof plastic sacks.
Structure
LAH violently reacts with water, including atmospheric moisture to liberate dihydrogen gas. The reaction proceeds according to the following idealized equation:
LiAlH4 + 4 H2O → LiOH + Al(OH)3 + 4 H2
This reaction can be used to generate hydrogen in the laboratory. Because they have absorbed enough moisture to produce a mixture of the white compounds lithium hydroxide and aluminium hydroxide, aged, air-exposed samples frequently appear white.
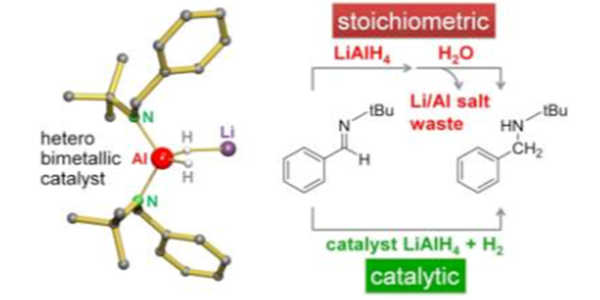
Preparation
LiAlH4 was first prepared from the reaction between lithium hydride (LiH) and aluminium chloride:
4 LiH + AlCl3 → LiAlH4 + 3 LiCl
In addition to this method, the industrial synthesis entails the initial preparation of sodium aluminium hydride from the elements under high pressure and temperature:
Na + Al + 2 H2 → NaAlH4
LiAlH4 is then prepared by a salt metathesis reaction according to:
NaAlH4 + LiCl → LiAlH4 + NaCl
which proceeds at a high rate of return LiCl is extracted from an ethereal solution of LAH via filtration, followed by precipitation of LiAlH4 to yield a product containing approximately 1% w/w LiCl.
An alternative preparation begins with LiH and metallic Al rather than AlCl3. The reaction proceeds well when dimethylether is used as a solvent and is catalyzed by a small amount of TiCl3 (0.2 percent). This method avoids salt cogeneration.
Applications
As a reducing agent, lithium aluminum hydride (LiAlH4) is widely used in organic chemistry. Because the Al-H bond is weaker than the B-H bond, it is more powerful than the related reagent sodium borohydride. It is used in the production of other chemicals, as a polymerization catalyst, as a source of hydrogen, and as a propellant. It is a powerful reducing agent that can be used in chemical synthesis to convert esters, carboxylic acids, acyl chlorides, aldehydes, epoxides, and ketones into alcohols.