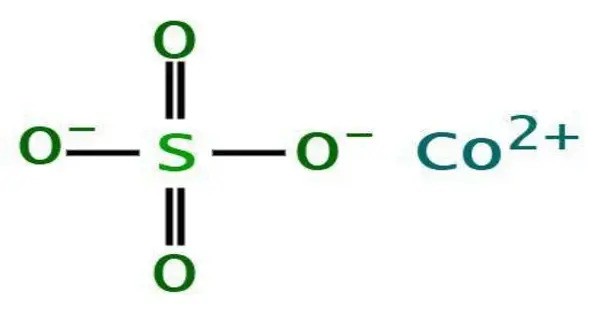
Cobalt(II) sulfate (CoSO₄) is an inorganic compound composed of cobalt, sulfur, and oxygen. It typically appears as red to pink crystalline solids, most commonly in the hydrated form CoSO₄·7H₂O, which is highly soluble in water. The anhydrous form is blue and is obtained by heating the hydrated crystals.
It is produced mainly by dissolving cobalt metal, cobalt(II) oxide, or cobalt(II) hydroxide in sulfuric acid. Cobalt(II) sulfate is an important precursor in the production of other cobalt compounds and cobalt-based pigments. It is widely used in electroplating to deposit cobalt metal coatings, which enhance wear and corrosion resistance. Its crystalline nature, vivid color, and solubility make it both industrially valuable and scientifically significant in coordination chemistry studies.
Properties
- Chemical formula: CoSO4·(H2O)n (n=0,1,6,7)
- Molar mass: 154.996 g/mol (anhydrous), 173.01 g/mol (monohydrate), 263.08 g/mol (hexahydrate), 281.103 g/mol (heptahydrate)
- Appearance: reddish crystalline (anhydrous, monohydrate), pink salt (hexahydrate)
- Odor: odorless (heptahydrate)
- Density: 3.71 g/cm3 (anhydrous), 3.075 g/cm3 (monohydrate), 2.019 g/cm3 (hexahydrate), 1.948 g/cm3 (heptahydrate)
- Melting point: 735 °C (1,355 °F; 1,008 K)
Preparation, and structure
It forms by the reaction of metallic cobalt, its oxide, hydroxide, or carbonate with aqueous sulfuric acid:
Co + H2SO4 + 7 H2O → CoSO4(H2O)7 + H2
CoO + H2SO4 + 6 H2O → CoSO4(H2O)7
The heptahydrate is only stable at humidity >70% at room temperature, otherwise it converts to the hexahydrate. The hexahydrate converts to the monohydrate and the anhydrous forms at 100 and 250 °C, respectively.
CoSO4(H2O)7 → CoSO4(H2O)6 + H2O
CoSO4(H2O)6 → CoSO4(H2O) + 5 H2O
CoSO4(H2O) → CoSO4 + H2O
The hexahydrate is a metal aquo complex consisting of octahedral [Co(H2O)6]2+ ions associated with sulfate anions (see image in table). The monoclinic heptahydrate has also been characterized by X-ray crystallography. It also features [Co(H2O)6]2+ octahedra as well as one water of crystallization.
Uses and reactions
Cobalt sulfates are important intermediates in the extraction of cobalt from its ores. Thus, crushed, partially refined ores are treated with sulfuric acid to give red-colored solutions containing cobalt sulfate.
In agriculture, cobalt(II) sulfate serves as a trace mineral supplement in animal feed, as cobalt is essential for vitamin B₁₂ synthesis in ruminants. In electrochemistry, it is employed in battery production and as a catalyst in certain chemical reactions.
Cobalt(II) sulfate is toxic in excessive amounts and can cause skin, respiratory, and systemic effects upon prolonged exposure. Due to its classification as a possible carcinogen, handling requires strict safety measures, including protective equipment and adequate ventilation.
Occurrences
Cobalt(II) sulfate is not abundant in nature as a free mineral but can occur as biebierite (CoSO₄·7H₂O) in oxidation zones of cobalt-rich ore deposits. It is mostly produced industrially by reacting cobalt metal, cobalt oxide, or cobalt carbonate with sulfuric acid. Naturally, it is found in areas where cobalt minerals (like cobaltite and erythrite) have been exposed to weathering and sulfate-rich environments.
Rarely, cobalt(II) sulfate is found in form of few crystallohydrate minerals, occurring among oxidation zones containing primary Co minerals (like skutterudite or cobaltite). These minerals are: biebierite (heptahydrate), moorhouseite (Co,Ni,Mn)SO4.6H2O, aplowite (Co,Mn,Ni)SO4.4H2O and cobaltkieserite (monohydrate).