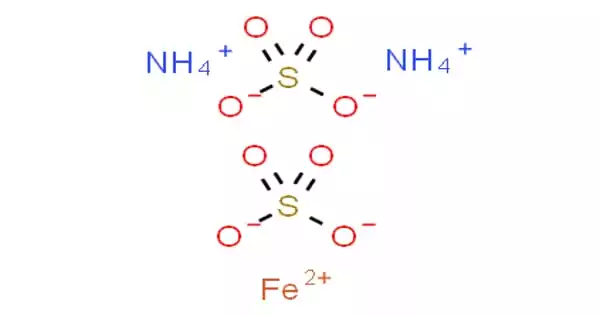
Mohr’s salt, or ammonium iron(II) sulfate, is an inorganic chemical having the formula (NH4)2Fe(SO4)2(H2O)6. It is classed as a double salt of ferrous sulfate and ammonium sulfate because it contains two distinct cations, Fe2+ and NH4+. It is a frequent laboratory reagent because it crystallizes easily and crystals resist air oxidation. Ferrous ammonium sulfate, like the other ferrous sulfate salts, dissolves in water to form the aquo complex [Fe(H2O)6]2+, which has octahedral molecular geometry. Mohrite is the mineral form of it.
Iron ammonium sulfate is used in titrations and colorimetric assays to assess oxidizing chemicals such as chlorine or the chemical oxygen demand in wastewater. For these analyses, the chemical is utilized to create Fe(II) standard solution. It is also a calibration standard in magnetic tests, a reducing agent, a polymerization catalyst, and is utilized in photographic chemistry.
Properties
- Melting point: 100 °C
- Density: 1.865
- Form: Liquid
- Color: pale green

Structure
This compound belongs to a class of double sulfates known as Schönites or Tutton’s salts. Tutton’s salts have the formula M2N(SO4)2.6H2O (M = different monocations) and produce monoclinic crystals. In terms of bonding, crystals are made up of octahedra [Fe(H2O)6]2+ centers that are hydrogen bound to sulfate and ammonium.
Structure of ferrous ammonium sulfate with emphasized hydrogen bonding network (N = violet, O = red, S = orange, Fe = big red). Mohr’s salt is named after the German scientist Karl Friedrich Mohr, who made significant breakthroughs in titration methodology in the nineteenth century.
Preparation
Mohr’s salt is prepared by dissolving an equimolar mixture of hydrated ferrous sulfate and ammonium sulfate in water containing a little sulfuric acid, and then subjecting the resulting solution to crystallization. Ferrous ammonium sulfate forms light green crystals. This salt when heated ionizes to give all cations and anions present in it.
Applications
This salt is the favored source of ferrous ions in analytical chemistry because it has a long shelf life and is resistant to oxidation. This stability extends to solutions that reflect the effect of pH on the ferrous/ferric redox pair to some extent. At high pH, this oxidation occurs more quickly. The ammonium ions make Mohr’s salt solutions mildly acidic, which slows the oxidation process. To minimize oxidation to ferric iron, sulfuric acid is routinely added to solutions.
It is employed in Fricke’s dosimeter to measure high gamma-ray dosages. Inhaling dust irritates the nose and throat. Ingestion causes mouth and stomach irritation. Dust irritates the eyes and might irritate the skin when in touch with it for an extended period of time.