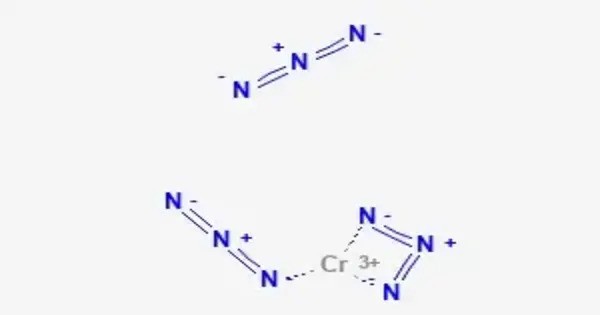
Chromium azide is an inorganic chemical compound with the formula Cr(N3)3. It is typically a coordination complex in which six azide ions (N₃⁻) coordinate to a central chromium atom. It is part of a class of compounds that are of interest mainly for their energetic properties, particularly in the context of explosives or pyrotechnic materials. This compound is known for its instability, and like other azide compounds, it can be sensitive to shock, heat, and friction, which can make it hazardous to handle.
It was separated in 1922 through the evaporation of a dry crystalline chromium(III) nitrate solution in absolute alcohol with sodium azide. Through a spectrophotometric study, it was shown that the chromium(III) nitrate solution’s green color was due to the mono-azido-chromium(III) complex.
Properties
Chromium azide formation has been investigated from chromium salts and sodium azide. Two It has luminescence properties from its optically active Cr3+ ions.
- Chemical formula: Cr(N3)3
- Molar mass: 178.06 g/mol
Reactivity
As a coordination compound, it contains the azide ion, which is a relatively strong nucleophile and can engage in various chemical reactions. Azides are known for their instability and can decompose to release nitrogen gas (N₂), sometimes with violent results.
Sensitivity
The compound is sensitive to physical shock, friction, or exposure to heat. Its explosive nature is one reason why it’s not commonly encountered outside of controlled, specialized chemical research environments.
Occurrences
Chromium azide is not a compound that occurs naturally in the environment. It is typically synthesized in the laboratory. Its creation generally involves the reaction of chromium salts, like chromium chloride (CrCl₃), with sodium azide (NaN₃) under carefully controlled conditions. Given its instability and sensitivity, chromium azide does not have common or widespread natural occurrences.
Uses
Chromium azide, like other metal azides, has potential applications in energetic materials, though its instability limits its practical use. It could be involved in pyrotechnics or as a component in detonators, where controlled decomposition is desired.