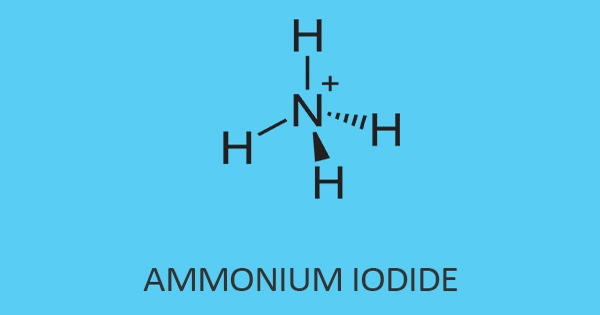
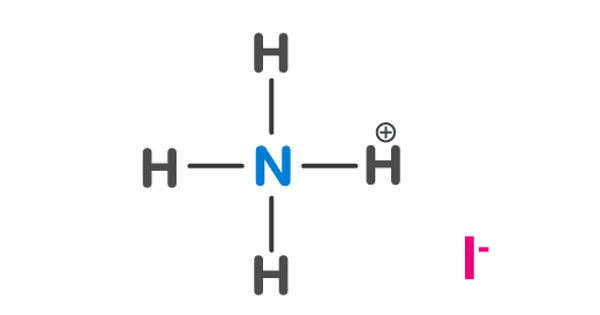
Ammonium iodate is an ionic compound consisting of one ammonium ion and one iodine ion. The formula for ammonium iodide is NH4I. It is an inorganic salt that is sparingly soluble in cold, and moderately soluble in hot water, like all iodate salts, it is a strong oxidizer. It is soluble in water, which means it disassociates when added to water.
Properties
The ammonium iodide is visible in the form of a white crystalline powder. In addition, it has a melting point of 551°C whereas, it has a boiling point of 405°C. Its molar mass is 144.94 g/mol with a density of 2.51 g/cm³. It is easily soluble when putting inside the water (H2O).
- Form: Crystalline
- Melting point: 150°C
- Density: 3.309
- Color: White
- Specific Gravity: 3.309
- Storage & Sensitivity: Moisture Sensitive and Ambient temperatures
- Solubility: Soluble in water.
Preparation
The preparation of the ammonium iodide takes place by the action of hydroiodic acid on the ammonia. Ammonium iodate can be obtained by neutralising a solution of iodic acid with ammonia.
HIO3 + NH3 → NH4IO3
Using its low solubility in water, it can also be precipitated from an iodate solution with an ammonium salt.
2 KIO3 + (NH4)2SO4 → 2 NH4IO3 + K2SO4
Unlike other iodates, ammonium iodate can’t be prepared by dissolving iodine in an ammonium hydroxide solution, instead the highly explosive nitrogen triiodide is formed.
3 I2 + 5 NH3 → 3 NH4I + NH3*NI3
It is also soluble when putting inside the solution of ethanol and then, as a result, it turns yellow on standing in the moist air, owing to decomposition with the process of liberation of the iodine.
Chemical properties
It can be prepared by the action of hydroiodic acid on ammonia. It is easily soluble in water, from which it crystallizes in cubes. Because ammonium iodate consists of the reducing ammonium ion and the oxidizing iodate ion, it already starts to decompose at 150 °C into nitrogen, oxygen, iodine and water.
NH4IO3 → ½N2 + ½O2 + ½I2 + 2H2O
Below 60 °C this reaction cannot sustain itself, but with catalysts like potassium dichromate or copper(II) chloride it can also combust at room temperature. It is also soluble in ethanol. It gradually turns yellow on standing in moist air, owing to decomposition with liberation of iodine.
Uses
Ammonium iodate is used as an oxidation reagent. Ammonium Iodate- 18O3 is the labeled analog of ammonium iodate. It is used in photographic chemicals and some medications.
Safety
Due to the inhalation of the ammonium iodide a person may suffer from multiple health issues, some of them are irritation of nose and throat. Contact of the ammonium iodide with the naked eyes of a living being can cause irritation in the eyes. Like all iodates, ammonium iodate is a strong oxidizer and should therefore be kept away from flammable materials like sulfur, phosphorus and metals powders. It should be kept in a tightly closed and protected container and the sunlight should not enter that container.
Information Source: