A pH indicator, also known as an acid-base indicator, is a compound that changes color in solution over a narrow pH range. To produce a visible color change, only a small amount of indicator compound is required. A pH indicator has no effect on the acidity or alkalinity of a chemical solution when used in a dilute solution.
A pH indicator is a halochromic chemical compound that is added to a solution in small amounts to determine the pH (acidity or basicity) of the solution visually. As a result, in the Arrhenius model, a pH indicator is a chemical detector for hydronium ions (H3O+) or hydrogen ions (H+). Normally, the indicator changes the color of the solution based on the pH. Indicators can also show change in other physical properties; for example, olfactory indicators show changes in their odor. The pH value of a neutral solution is 7.0 at 25°C (standard laboratory conditions).
pH indicators are weak acids that exist as natural dyes and use color change to indicate the concentration of H+ (H3O+) ions in a solution. A pH value is calculated from the negative logarithm of this concentration and is used to indicate whether the substance being tested is acidic, basic, or neutral.
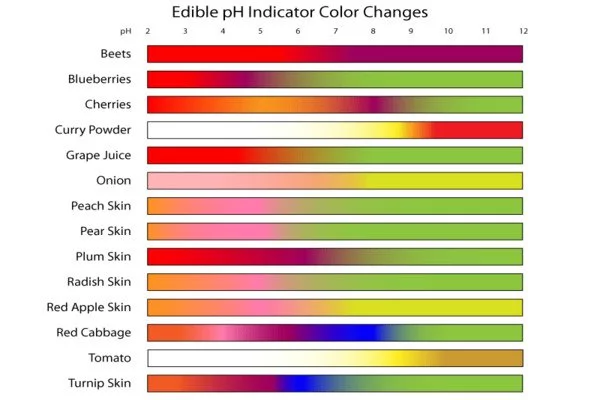
Solutions with pH values less than 7.0 are considered acidic, while solutions with pH values greater than 7.0 are considered basic. Because most naturally occurring organic compounds, such as carboxylic acids and amines, are weak electrolytes, pH indicators have numerous applications in biology and analytical chemistry. Furthermore, pH indicators are one of three major types of indicator compounds used in chemical analysis. For the quantitative analysis of metal cations, the use of complexometric indicators is preferred, whereas the third compound class, the redox indicators, are used in redox titrations (titrations involving one or more redox reactions as the basis of chemical analysis).
Application
Titrations in analytical chemistry and biology frequently use pH indicators to determine the extent of a chemical reaction. Because of the subjective nature of color selection (determination), pH indicators are prone to inaccurate readings. A pH meter is commonly used in applications that require precise pH measurement. A combination of different indicators is sometimes used to achieve several smooth color changes over a wide pH range. These commercial indicators (for example, universal indicator and Hydrion papers) are used when only a rough idea of pH is required.
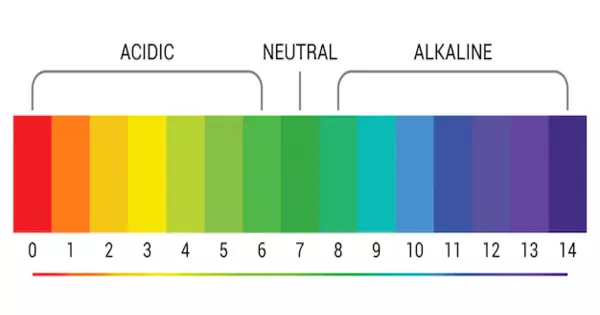
Several common laboratory pH indicators are listed below. At pH values within the listed transition range, indicators typically exhibit intermediate colors. For example, phenol red turns orange between pH 6.8 and pH 8.4. The transition range may vary slightly depending on the indicator concentration in solution and the temperature at which it is used. The diagram on the right depicts indicators and their operational ranges as well as color changes.
pH indicators are tailored to the pH range that is desired. Common indicators, such as phenolphthalein, methyl red, and bromothymol blue, are used to indicate pH ranges of 8 to 10, 4.5 to 6, and 6 to 7.5, respectively. On these scales, phenolphthalein changes from colorless to pink, methyl red changes from red to yellow, and bromothymol blue changes from yellow to blue. The pH range for universal indicators, on the other hand, is much wider, and the number of color changes is much greater.