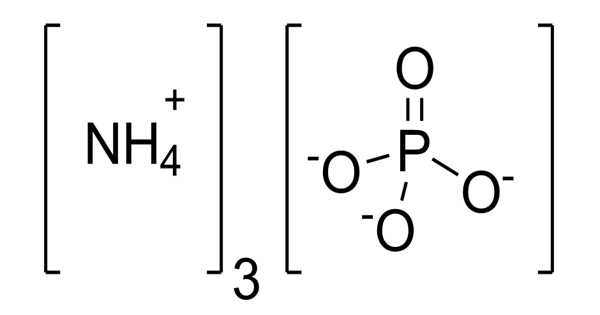
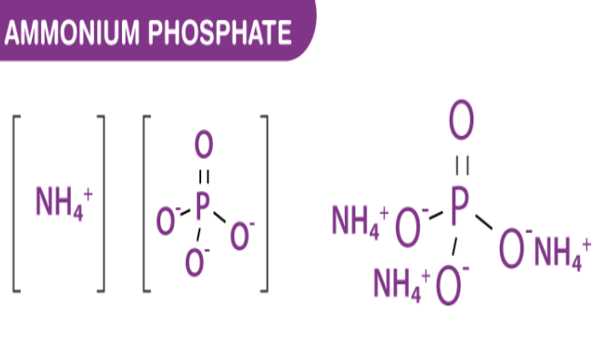
The inorganic compound ammonium phosphate has the formula (NH4)3PO4. It is ammonia and phosphoric acid salt. Ammonium cations and phosphate anion make up this compound. It is the orthophosphoric acid ammonium salt. It is soluble in water, and when boiled, the aqueous solution loses ammonia and forms acid phosphate (NH4)(H2PO4).
(NH4)3PO4 is a related “double salt.” (NH4)2HPO4 is also recognized, but its use is impractical. Ammonia is produced by both triammonium salts. It is obtained as a crystalline powder by combining concentrated ammonia and phosphoric acid solutions, or by adding an excess of ammonia to the acid phosphate (NH4)2 (HPO4). Unlike triammonium salts, which are unstable, diammonium phosphate (NH4)2HPO4 and monoammonium salt (NH4)H2PO4 are stable materials that are commonly used as fertilizers to provide plants with fixed nitrogen and phosphorus.
Ammonium phosphate is the salt of ammonia and phosphoric acid. It consists of ammonium cations and phosphate anion. It is obtained as a crystalline powder upon mixing concentrated solutions of ammonia and phosphoric acid.
Properties
Ammonium phosphate is made by combining ammonium phosphate and urea in a molten state. Significant heat is generated, causing the ammonium phosphate sulphate to become molten. It is a class of nitrogen phosphorus materials that include monoammonium phosphates and diammonium phosphates, as well as mixtures of the two and combinations with ammonium nitrate or ammonium sulfate.
- Density: 1.619 g/cubic cm
- Molecular Weight/ Molar Mass: 149.09 g/mol
- Boiling Point: 130°C
- Melting Point: 155 °C (311 °F; 428 K) decomposes
- Odour: Ammonia odour
- Appearance: White, tetrahedral crystals
- Covalently-Bonded Unit: 3
- Solubility: Readily soluble in water
Preparation of triammonium phosphate
Ammonium phosphate is a compound with two replaceable hydrogen atoms, whereas monobasic ammonium phosphate has only one hydrogen atom to donate to the base during the acid-base reaction of ammonium and phosphate.
Triammonium phosphate can be prepared in the laboratory by treating 85% phosphoric acid with 30% ammonia solution:
H3PO4 + 3 NH3 → (NH4)3PO4
(NH4)3PO4 is a colorless, crystalline solid. The solid, which has the odor of ammonia, is readily soluble in water. The salt converts to diammonium hydrogen phosphate (NH4)2HPO4.
Uses
As a high source of elemental nitrogen, ammonium phosphate is used as an ingredient in some fertilizers. It is also used in thermoplastic compositions as a flame retardant. It is found in intumescent paints and mastics, where it acts as an acid catalyst.
Furthermore, fertilizers containing ammonium phosphate frequently aid in the addition of nitrogen to the surrounding soil, thereby promoting growth and root development. Its solubility allows for rapid release into soils, which benefits newer plants and plants that need to be revitalized.