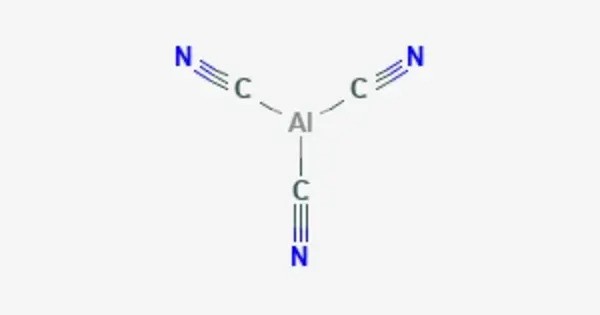
Aluminium cyanide is a metallic cyanide with a chemical formula of Al(CN)3. It is a white solid that undergoes hydrolysis to produce aluminium hydroxide and hydrogen cyanide. It has the molecular formula Al(CN)₃. In this compound, aluminum is bonded to three cyanide ions (CN⁻), and it is usually described as aluminum(III) cyanide because aluminum typically forms a +3 oxidation state in its compounds.
It is a chemical with interesting properties due to its toxicity and reactivity, but it doesn’t occur naturally in significant quantities and has limited uses in industrial settings.
Properties
It typically forms a polymeric structure where the aluminum atoms are surrounded by cyanide ions. The compound is highly toxic and can release toxic cyanide gas (HCN) if decomposed or exposed to heat. Like many cyanide compounds, it is dangerous to handle due to the potential for poisoning.
- Chemical formula: C3AlN3
- Molar mass: 105.036 g·mol−1
- Appearance: white solid
- Solubility in water: Reacts
- Molecular Weight: Approximately 115.01 g/mol.
- Solubility: It is soluble in water, which can lead to the formation of hydrocyanic acid (HCN) and the release of cyanide ions under certain conditions.
Synthesis
Aluminium cyanide was first produced in 1924 as its ammoniate, Al(CN)3·5NH3, by reacting aluminium metal and mercury(II) cyanide in liquid ammonia to prevent hydrolysis.
2 Al + 3 Hg(CN)2 → 2 Al(CN)3 + 3 Hg
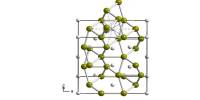
When the ammoniate contacts water, it produces aluminium hydroxide, ammonia, and ammonium cyanide. The compound was produced in 2001 by the reaction of lithium tetrachloroaluminate and trimethylsilyl cyanide in diethyl ether. Its atoms form a lattice, and X-ray crystallography shows that its crystals form an octahedral Prussian-blue-like structure.
Reactivity
- Highly Toxic: The cyanide ions in the compound are highly toxic, as cyanide interferes with the body’s ability to use oxygen. Handling should be done with care.
- Decomposition: Upon heating, it may decompose to produce toxic gases, including HCN.
- Stability: It’s relatively unstable, and the compound is usually unstable in the presence of moisture or acids, as it may release toxic cyanide vapors.
Occurrences
Synthesis: Aluminum cyanide is not typically found in nature but can be synthesized under controlled laboratory conditions. One common way it can be made is by reacting aluminum metal with cyanogen chloride (CNCl) or potassium cyanide (KCN) in a controlled environment.
In Industrial Processes: While aluminum cyanide is not a major industrial product, cyanide chemistry plays a significant role in industries such as mining (gold extraction, for instance) and in the synthesis of organic compounds. However, Al(CN)₃ itself is not directly used in most industrial applications.
Safety Concerns
Due to the presence of cyanide, handling aluminum cyanide requires careful safety measures. Cyanide compounds can cause severe toxicity if ingested, inhaled, or absorbed through the skin, leading to poisoning or even death in high enough concentrations.