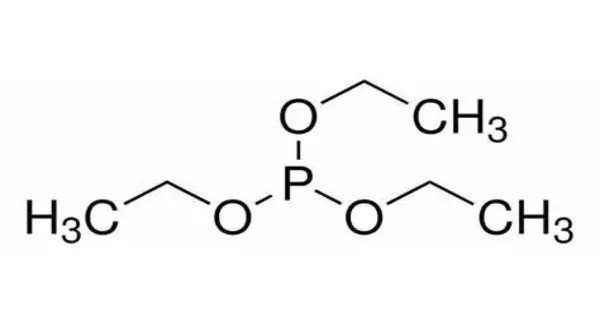
Triethyl phosphite (TEP) is an organophosphorus compound, specifically a phosphite ester, with the formula P(OCH2CH3)3, often abbreviated P(OEt)3. It’s a clear, colorless liquid that is primarily used in organic synthesis and as a precursor for various chemical compounds. It is a colorless, malodorous liquid.
It is a colorless liquid with a faint odor and is often used as a reagent in chemical synthesis. It is used as a ligand in organometallic chemistry and as a reagent in organic synthesis. It is flammable and can be hazardous if it comes into contact with skin or is inhaled.
Triethylphosphite is prepared by treating phosphorus trichloride with ethanol in the presence of a base, typically a tertiary amine:
PCl3 + 3 EtOH + 3 R3N → P(OEt)3 + 3 R3NH + 3 Cl−
In the absence of the base, the reaction of ethanol and phosphorus trichloride affords diethylphosphite ((EtO)2P(O)H). Of the many related compounds can be prepared similarly, triisopropyl phosphite is an example (b.p. 43.5 °C/1.0 mm; CAS# 116-17-6).
Properties
- Chemical formula: C6H15O3P
- Molar mass: 166.157 g·mol−1
- Appearance: colorless liquid
- Density: 0.969 g/mL
- Melting point: −70 °C (−94 °F; 203 K)
- Boiling point: 156 °C (313 °F; 429 K) (57 to 58 °C at 16 mm)
- Solubility: soluble in most organic solvents
Uses
- As a reagent in organic synthesis: Triethyl phosphite is frequently used in the synthesis of phosphorus-containing compounds, including phosphonates, phosphines, and various other organophosphorus derivatives.
- In the production of flame retardants: It is used in the creation of phosphorous-based flame retardants.
- In the preparation of pesticides: It plays a role in the synthesis of certain agrochemicals.
- As a stabilizer in various chemical processes.
Occurrences
Triethyl phosphite is mainly synthesized for industrial use and does not occur naturally in significant quantities. Its production typically involves the reaction of phosphorus trichloride with ethanol. It is used in various synthetic processes, including:
As a Reagent in Organic Synthesis:
- Triethyl phosphite is commonly used in the phosphorylation of alcohols, synthesis of phosphonates, and as a precursor in the formation of phosphoric esters.
- It is involved in the Staudinger reaction, which is useful for the formation of amides from imines.
In the Synthesis of Organophosphorus Compounds:
- Triethyl phosphite is used in the synthesis of other phosphorus-containing compounds like phosphonates, phosphoramidates, and organophosphorus pesticides.
- It is an important starting material for phosphorus-based flame retardants and plasticizers.
- In the Production of Phosphorous Acids:
- It is used in the industrial production of phosphorous acid and other phosphorous derivatives.
- In the Pharmaceutical Industry:
- It is used to synthesize certain pharmaceutical compounds and intermediates.
Safety
- Triethyl phosphite is flammable and can be hazardous if it comes into contact with skin or is inhaled.
- It can release toxic gases, such as phosphine, when heated or in contact with water or moisture.
- Proper handling with personal protective equipment (PPE) is essential to prevent exposure.