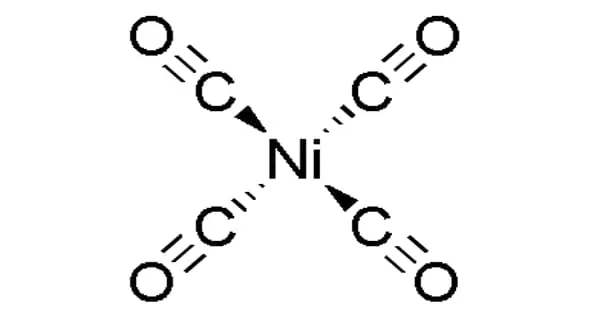
The organonickel compound nickel carbonyl has the formula Ni(CO)4. It is a metal carbonyl and a nickel coordination entity. It is a clear colorless to yellow volatile liquid that is flammable and emits a yellow flame when burned. This colorless liquid is nickel’s primary carbonyl. It has a higher density than water and is insoluble in water, but it is soluble in alcohol, benzene, chloroform, acetone, ethanol, carbon tetrachloride, and nitric acid.
It is a reagent in organometallic chemistry and an intermediate in the Mond process for producing very high-purity nickel, though the Mond Process has fallen out of favor due to the health risks associated with working with the compound.
Nickel carbonyl is one of the most dangerous substances yet encountered in nickel chemistry due to its very high toxicity, compounded with high volatility and rapid skin absorption.
Properties
It is a toxic, colorless liquid, with a melting point of 248 degrees Kelvin, and a boiling point of 316 degrees Kelvin. Its vapors form an explosive mixture with air. It reacts with halogens, in an organic solvent, and form dai halides. It reacts with sulphuric acid and liberates carbon monoxide.
- Molecular Weight: 170.73
- Appearance: Colorless Liquid
- Melting Point: -19.3 °C
- Boiling Point: 43 °C
- Density: 1.32 g/mL
- Form: liquid
- Color: colorless

Structure and bonding
The oxidation state of nickel in nickel tetracarbonyl is zero. The formula adheres to the 18-electron rule. The molecule has four carbonyls (carbon monoxide) ligands and is tetrahedral. This molecule has been subjected to electron diffraction studies, and the Ni–C and C–O distances have been calculated to be 1.838(2) and 1.141(2) angstroms, respectively.
Preparation
Ludwig Mond created Ni(CO)4 by directly reacting nickel metal with carbon monoxide in 1890. This groundbreaking work foreshadowed the existence of numerous other metal carbonyl compounds, including vanadium, chromium, manganese, iron, and cobalt. By the end of the nineteenth century, it was also being used in the industrial purification of nickel.
Carbon monoxide is passed over impure nickel at 323 K (50 °C; 122 °F). At 130 °C, the rate is optimal.
- By direct combination: It is prepared by passing carbon monoxide at one atmospheric pressure over freshly reduced nickel at room temperature.
- By reductive carbonylation: It is made by reacting nickel iodide or sulphide with carbon monoxide at a pressure of 200 atmospheres and a temperature of 473 degrees Kelvin in the presence of a reducing agent, such as copper.
- Nickel one cyanide disproportionation: Potassium tetra cyano nickel is first reduced to Nickel one complex by potassium amalgam. At 264 degrees Kelvin, this is treated with carbon monoxide to form potassium hexa cyano dai carbonyl Nickel, which disproportionates with water or acid to form Nickel tetra carbonyl.
Production Methods
Carbon monoxide and nickel metal react to form nickel carbonyl. It can also form as a byproduct in industrial processes that use nickel catalysts, such as coal gasification, crude oil refining, and hydrogenation reactions (293). Conditions for its formation occur when carbon monoxide comes into contact with an active form of nickel at elevated pressures and temperatures ranging from 50 to 150°C.
Laboratory routes
Ni(CO)4 is not widely available on the market. It is easily produced in the lab by carbonylation of commercially available bis(cyclooctadiene)nickel (0). It can also be made by reducing ammoniacal nickel sulfate solutions with sodium dithionite in a CO atmosphere.
Uses
Nickel carbonyl is used in nickel vapoplating processes in the metallurgical and electronics industries, as well as in the catalytic synthesis of methyl- and ethyl acrylate monomers. It was used for many years to produce pure nickel via the Mond process, which has been considered obsolete since around 1970.
The vapors are denser than the air. Nickel carbonyl is used in industries to nickel coat steel and other metals, as well as to produce very pure nickel. As a solid deposit, nickel carbonyl is peroxidized by air and decomposes to ignite.