Researchers from the Faculty of Science at Charles University have examined the complex relationship between anaerobic amoebae from the genus Pelomyxa and their prokaryotic partners in a recent study. They worked with researchers from the Max-Planck Institute for Terrestrial Microbiology and European Molecular Biology Laboratory from Heidelberg.
Pelomyxa, known for their unique symbiotic relationships with multiple prokaryotic endosymbionts, has long mystified scientists regarding the role these symbionts play in their host’s metabolism.
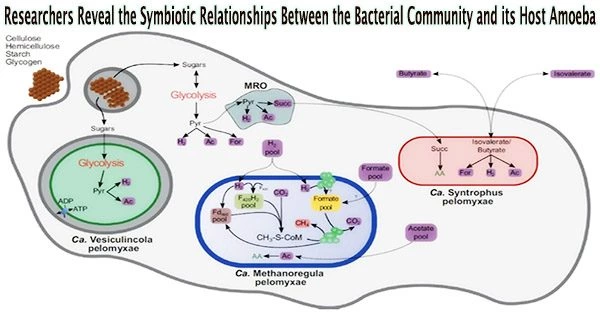
The study team has revealed the mysteries of the prokaryotic community thriving inside Pelomyxa schiedti using single-cell genomics and transcriptomics. This symbiotic consortium is comprised of two distinct bacteria, Candidatus Syntrophus pelomyxae (class Deltaproteobacteria) and Candidatus Vesiculincola pelomyxae (class Clostridia), along with a methanogen known as Candidatus Methanoregula pelomyxae.
The study, which was just published in The ISME Journal, provides information on how these symbionts are distributed throughout the host amoeba. In contrast to the other endosymbionts, which were found to be evenly dispersed throughout the cytosol, Candidatus Vesiculincola pelomyxae was shown to be localized within vesicles. Interestingly, Candidatus Methanoregula pelomyxae was enriched around the nucleus.
This study reveals the fascinating strategies employed by these symbiotic organisms to thrive together. While we know of many symbioses between eukaryotes and archaeal methanogens, this is the first example where the methanogenic symbiont is obligate. We are almost certain that similar metabolic couplings with a central role for methanogenesis are very common in anaerobic environments, only the actors are changing.
Dr. Treitli
The metabolic methods of these symbiotic partners were identified by a thorough examination of their genomes and transcriptomes. It is anticipated that Pelomyxa schiedti’s cellulolytic activity would result in the production of simple sugars that will power both the organism’s own metabolism and that of Candidatus Vesiculincola pelomyxae.
In contrast, Candidatus Syntrophus pelomyxae’s energy metabolism depends on the breakdown of butyrate and isovalerate from the environment.
Notably, hydrogenases, a mechanism reliant on low hydrogen partial pressure, were used by both bacteria and the amoeba to aid in the transfer of electrons. Candidatus Methanoregula pelomyxae, which used H2 and formate for methanogenesis, accomplished this important control.
To comprehend the necessity of these symbiotic connections, the researchers ran tests. The use of 2-bromoethanesulfonate, a particular inhibitor of methanogenesis, proved lethal for the amoebae while the bacterial symbionts were efficiently eradicated by vancomycin therapy without compromising the vitality of the amoebae. This emphasized how crucial methanogenesis was to the survival of this complex consortium.
A member of the research team, Dr. Treitli from the Faculty of Science of Charles University, said, “This study reveals the fascinating strategies employed by these symbiotic organisms to thrive together. While we know of many symbioses between eukaryotes and archaeal methanogens, this is the first example where the methanogenic symbiont is obligate. We are almost certain that similar metabolic couplings with a central role for methanogenesis are very common in anaerobic environments, only the actors are changing.”
This study not only advances our understanding of symbiosis but also has potential use in a variety of disciplines, from microbiology to ecological research. The research deepens our understanding of the delicate balance that supports ecosystems and demonstrates how intricate and linked life forms are.