University of California San Diego nano engineers have created a novel and potentially more effective method of delivering messenger RNA (mRNA) into cells. Their method entails putting mRNA within nanoparticles that resemble the flu virus – a naturally efficient carrier for transporting genetic material like RNA inside cells. A report on the new mRNA delivery nanoparticles was just published in the journal Angewandte Chemie International Edition.
The research addresses a significant difficulty in the field of drug delivery: reliably delivering big biological drug molecules into cells while shielding them from endosomes. Inside the cell, these tiny acid-filled bubbles act as barriers, trapping and digesting bigger molecules that try to enter. Once inside the cell, biological therapies require a mechanism to exit the endosomes in order to perform their functions.
“Because current mRNA delivery systems lack effective endosomal escape mechanisms, the amount of mRNA that really enters cells and has an effect is quite low. When they are administered, the vast majority of them are squandered “said senior author Liangfang Zhang, a nanoengineering professor at UC San Diego’s Jacobs School of Engineering.
If you can get more mRNA into cells, you can take a much lower dose of an mRNA vaccine, which could lessen adverse effects while maintaining efficiency.
Liangfang Zhang
According to Zhang, achieving effective endosomal escape would be a game-changer for mRNA vaccines and therapeutics. “If you can get more mRNA into cells, you can take a much lower dose of an mRNA vaccine, which could lessen adverse effects while maintaining efficiency.” It may also boost the delivery of short interfering RNA (siRNA) into cells, which is utilized in some forms of gene therapy.
Viruses are highly good at evading the endosome in nature. The influenza virus A virus, for example, has a unique protein called hemagglutinin on its surface that, when activated by acid inside the endosome, causes the virus’s membrane to merge with the endosomal membrane. The endosome is therefore opened, allowing the virus to release its genetic material into the host cell without being killed.
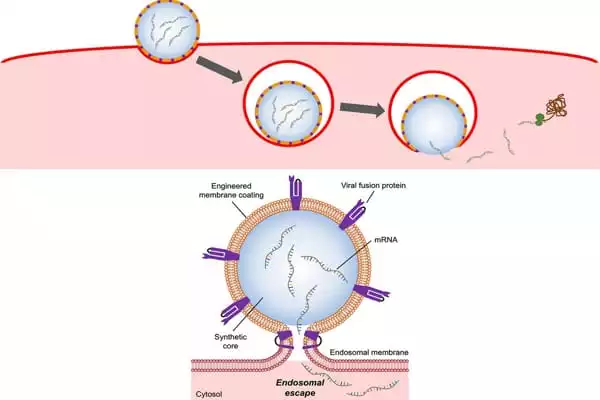
Zhang and his colleagues created mRNA delivery nanoparticles that mirror the capabilities of the flu virus to do so. The nanoparticles were created by genetically engineering cells in the lab to express the hemagglutinin protein on their cell membranes. They next removed the membranes from the cells, breaking them into tiny bits and coating them on nanoparticles comprised of a biodegradable polymer pre-packed with mRNA molecules.
The end result is a flu virus-like nanoparticle that can enter a cell, break free from the endosome, and allow its mRNA payload to accomplish its job: direct the cell to manufacture proteins.
The influenza virus’s genome contains a negative-sense RNA. To reproduce, the virus must first create a positive sense mRNA in order to produce the essential enzymes. After the enzymes have been translated, replication can begin. The enzymes are then used to create positive sense cRNA from the original negative-sense RNA. The positive sense cRNA is subsequently used to create negative sense RNA progeny. Finally, the viral progeny sprout from the host cell, ready to infect new cells.
The influenza virus penetrates the host cell by binding its hemagglutinin to the sialic acid present on the host’s glycoproteins or glycolipid receptors. The virus is then endocytosed by the cell. The virus changes form and merges its envelope with the endosomal membrane in the acidic environment of the endosomes. This is followed by a signal that causes the viral nucleocapsid to be released into the host cytoplasm. The nucleocapsid then goes to the host nucleus.
The nanoparticles were tested in mice by the researchers. The nanoparticles contained mRNA expressing Cypridina luciferase, a bioluminescent protein. They were given to the mice both through the nose (droplets of a nanoparticle-containing solution injected to the nostrils) and intravenously. The researchers examined the mice’s nostrils and blood and discovered a substantial amount of bioluminescence signal. This demonstrated that the flu virus-like nanoparticles transported their mRNA payloads into cells in vivo.
The researchers are currently testing their technology for therapeutic mRNA and siRNA payload delivery.