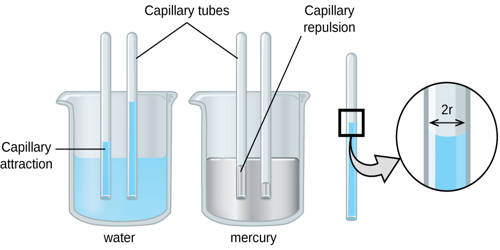
Capillary action is the ability of a liquid to flow in narrow spaces without the assistance of, or even in opposition to, external forces like gravity. It helps bring water up into the roots. The effect can be seen in the drawing up of liquids between the hairs of a paint-brush, in a thin tube, in porous materials such as paper and plaster, in some non-porous materials such as sand and liquefied carbon fiber, or in a biological cell. It occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. Capillary action was first recorded by Leonardo da Vinci. Albert Einstein’s first scientific paper in 1900 was written on the subject of capillarity.
Capillary action is important for moving water around. It is defined as the movement of water within the spaces of a porous material due to the forces of adhesion, cohesion, and surface tension. It occurs because of intermolecular forces between the liquid and surrounding solid surfaces. It is caused by the combination of cohesive forces of the liquid and the adhesive forces between the liquid and tube material. If the diameter of the tube is sufficiently small, then the combination of surface tension (which is caused by cohesion within the liquid) and adhesive forces between the liquid and container wall act to propel the liquid. Examples of capillary action include the uptake of water in paper and plaster, the wicking of paint between the hairs of a paintbrush, and the movement of water through sand.
Three main variables that determine whether a liquid possesses capillary action are:
- Cohesive force: It is the intermolecular bonding of a substance where its mutual attractiveness forces them to maintain a certain shape of the liquid.
- Surface tension: This occurs as a result of like molecules, cohesive forces, banding together to form a somewhat impenetrable surface on the body of water.
- Adhesive force: When forces of attraction between unlike molecules occur, it is called adhesive forces.
In plants and animals
Capillary action is seen in many plants. Water is brought high up in trees by branching; evaporation at the leaves creating depressurization; probably by osmotic pressure added at the roots; and possibly at other locations inside the plant, especially when gathering humidity with air roots.
Capillary action for the uptake of water has been described in some small animals, such as Ligia exotica and Moloch horridus. It is also essential for the drainage of constantly produced tear fluid from the eye. Two tiny-diameter tubes, the lacrimal ducts, are present in the inner corner of the eyelid; these ducts secrete tears into the eye. It is the movement of water in and out of your cellular structure that deposits vitamins, nutrients, and vital blood plasma.