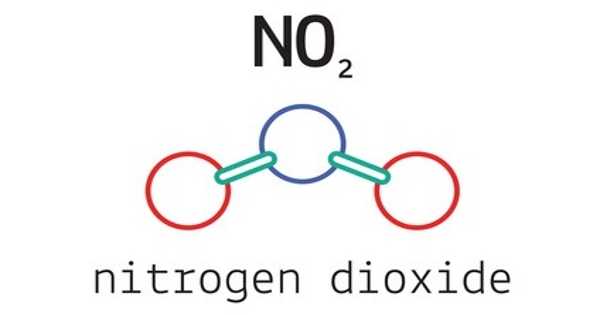
Nitrogen dioxide, also known as NO2, is a chemical compound with the formula NO2. When cooled or compressed, it appears as a reddish-brown gas or a yellowish-brown liquid. It is one of a number of nitrogen oxides. NO2 is an intermediate in the industrial synthesis of nitric acid, which is produced in millions of tons per year for use primarily in the production of fertilizers. At higher temperatures, it transforms into a reddish-brown gas. If inhaled in large quantities, it can be fatal.
Nitrogen dioxide is released into the atmosphere by vehicles, power plants, industrial emissions, and off-road sources such as construction, lawn, and gardening equipment. All of these sources are powered by fossil fuels. NO2 is produced when high-temperature fossil fuels such as coal, oil, gas, or diesel are burned. The presence of NO2 and other nitrogen oxides in the outdoor air contributes to particle pollution as well as the chemical reactions that produce ozone.
Properties
Nitric oxide is colorless and oxidizes to nitrogen dioxide in the atmosphere. Nitrogen dioxide has an unpleasant odor and is an acidic, highly corrosive gas that can harm our health and the environment.
Nitrogen dioxide is a reddish-brown gas with a pungent, acrid odor above 21.2°C (70.2°F; 294.3 K), a yellowish-brown liquid below 21.2°C (70.2°F; 294.3 K), and a colorless dinitrogen tetroxide (N2O4) below 11.2°C (11.8°F; 261.9 K).
Unlike ozone, O3, nitrogen dioxide’s ground electronic state is a doublet state because nitrogen has one unpaired electron, which reduces the alpha effect compared to nitrite and creates a weak bonding interaction with the oxygen lone pairs. Because the lone electron in NO2 indicates that the compound is a free radical, the formula for nitrogen dioxide is frequently written as •NO2.
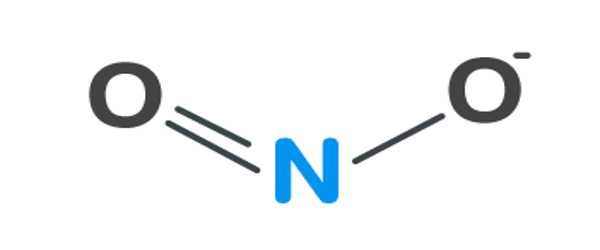
Preparation and reactions
Nitrogen dioxide typically arises via the oxidation of nitric oxide by oxygen in the air:
2 NO + O2 → 2 NO2
Nitrogen dioxide is formed in most combustion processes using air as the oxidant. At elevated temperatures, nitrogen combines with oxygen to form nitric oxide: O2 + N2 → 2 NO
In the laboratory, NO2 can be prepared in a two-step procedure where dehydration of nitric acid produces dinitrogen pentoxide, which subsequently undergoes thermal decomposition:
2 HNO3 → N2O5 + H2O2
N2O5 → 4 NO2 + O2
The thermal decomposition of some metal nitrates also affords NO2:
2 Pb(NO3)2 → 2 PbO + 4 NO + O2
Alternatively, reduction of concentrated nitric acid by a metal (such as copper).
4 HNO3 + Cu → Cu(NO3)2 + 2 NO2 + 2 H2O
Or finally, by adding concentrated nitric acid over the tin, a hydrated stannic oxide is produced as a byproduct.
4 HNO3 + Sn → H2O + H2SnO3 + 4 NO2
Uses
NO2 is used as an intermediate in the production of nitric acid, as a nitrating agent in the production of chemical explosives, as a polymerization inhibitor for acrylates, as a flour bleaching agent, and as a sterilization agent at room temperature. It is also used as an oxidizer in rocket fuel, such as red fuming nitric acid; it was used in the Titan rockets that launched Project Gemini, in the Space Shuttle’s maneuvering thrusters, and an unmanned space probes sent to various planets.
Precaution
Nitrogen oxide is an extremely toxic gas. Exposure causes lung inflammation, which may cause only minor discomfort or go unnoticed, but the resulting edema may cause death several days later. Toxic by inhalation (vapor) and absorption through the skin. Noncombustible, but hastens the combustion of combustible materials.