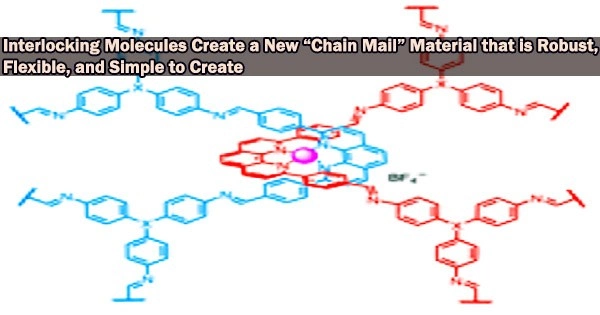
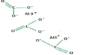
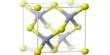
Chemists at the University of California, Berkeley have developed a novel material made of millions of identical, interlocking molecules that, for the first time, enables the synthesis of large-scale 2D or 3D structures that are flexible, strong, and resilient, similar to the chain mail that shielded medieval knights.
The material, called an infinite catenane, can be synthesized in a single chemical step.
French chemist Jean-Pierre Sauvage shared the 2016 Nobel Prize in Chemistry for synthesizing the first catenane two linked rings. These structures laid the groundwork for the development of molecular machines, or molecular structures that can move.
But the chemical synthesis of catenanes has remained laborious. Each additional ring that is added to a catenane requires a new chemical synthesis to create. Chemists have only succeeded in creating 130 interwoven rings in numbers too small to view without an electron microscope in the 24 years after Sauvage first discovered a two-ring catenane.
The new type of catenane, produced in the laboratory of Omar Yaghi, UC Berkeley professor of chemistry, can be produced with an unlimited number of linked units in three dimensions. The structures are flexible enough to bend without breaking because the constituent pieces mechanically interlock rather than being joined by chemical bonds.
“We think that this has really important implications, not just in terms of making tough materials that don’t fracture, but also materials that would go into robotics and aerospace and armored suits and things like this,” said Yaghi, the James and Neeltje Tretter Chair Professor of Chemistry, co-director of the Kavli Energy NanoSciences Institute and the California Research Alliance by BASF, and chief scientist at UC Berkeley’s Bakar Institute of Digital Materials for the Planet.
Yaghi and his colleagues, including first author Tianqiong Ma, a UC Berkeley postdoctoral fellow, reported details of the chemical process this week in the journal Nature Synthesis.
Traditionally, this interlocking has been done through a multistep, arduous process to make only molecules that have one or two or three interlocking rings, or polyhedra. But to make materials that have amazing properties, like toughness and resiliency, you need millions and millions of these interlockings to be made.
Omar Yaghi
Reticular chemistry
The leap forward in catenane production is possible using a type of chemistry that Yaghi invented more than 30 years ago: reticular chemistry. He describes it as “stitching molecular building blocks into crystalline, extended structures by strong bonds.”
The metal-organic frameworks (MOFs) and covalent organic frameworks (COFs) he created with this method are affordable porous materials that are excellent for catching, storing, or separating gases like carbon dioxide, hydrogen, and water vapor. More than 100,000 varieties of MOFs have been made to date.
Simply create the appropriate hybrid molecules metal clusters bound to an organic ligand and combine them with a solution to cause them to bind together and form a stiff, extremely porous 3D network. According to temperature, specific molecules are chosen for the chemical groups inside the framework to attach and release, while rejecting others.
One MOF that Yaghi created can pull water from even the driest air and then release it when heated, allowing water capture in deserts.
To make catenanes, Yaghi and Ma synthesized a molecule with a crossing between two identical halves, covalently linked by a copper atom. The structure, which they refer to as a catena-COF, resembles two connected boomerangs that cross at a copper atom.
These molecules combine to produce a porous, three-dimensional network of interconnecting building pieces. Adamantane, one of the building components, is a polyhedral molecule whose six arms basically lock together to form an expanded framework.
“What’s new here is that the building units have these crossings, and because of the crossings, you get interlocking systems that have interesting, flexible and resilient properties,” Yaghi said. “They’re programed to come together in one step. That’s the power of reticular chemistry. Instead of building them up one unit at a time to make the larger structure, you actually have them programmed such that they come together and grow on their own.”
The molecule with a crossing can be chemically altered so that the final catenane interacts with specific compounds. Yaghi calls these materials(∞) catenanes,, using the symbol for infinity.
“I think that is a first step towards making materials that can flex and potentially can stiffen in response to stimuli, like a particular motion,” he said. “So, in certain orientations, it could be very flexible, and in certain other orientations, it could become stiff, just because of the way the structure is built.”
He pointed out that although on a microscopic level these catenanes extend in three directions, they may be manufactured thin enough to be used in two dimensions, such as in clothes. Recently, some scientists have reported that they have created MOFs and COFs by 3D printing, so it may be possible to 3D print catenanes, as well, much like weaving a cloth.
“Traditionally, this interlocking has been done through a multistep, arduous process to make only molecules that have one or two or three interlocking rings, or polyhedra. But to make materials that have amazing properties, like toughness and resiliency, you need millions and millions of these interlockings to be made,” he said.
“The traditional way of making them just doesn’t cut it. And reticular chemistry comes in with the building block approach and finds a way of doing it in one step. That’s really the power of this report.”
The work was partly supported by King Abdulaziz City for Science and Technology and the Defense Advanced Research Projects Agency (DARPA, HR001-119-S-0048). The researchers used resources of the Advanced Light Source at Lawrence Berkeley National Laboratory (DOE DE-AC02-05CH11231).