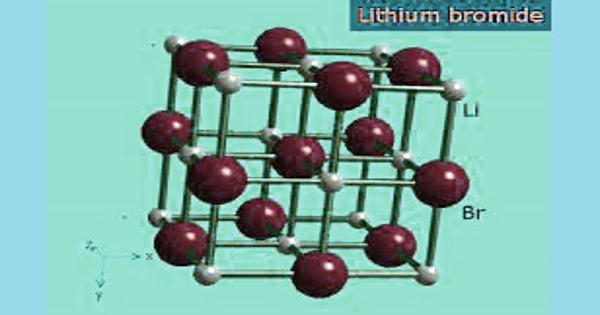
Lithium bromide (LiBr) is a chemical compound of lithium and bromine. It is an extremely hygroscopic chemical commonly used in absorption chillers, where water is the refrigerant. Its extreme hygroscopic character makes LiBr useful as a desiccant in certain air conditioning systems. Because of its high affinity to water, LiBr can easily absorb excess vapor, making the system an environmentally friendly non-CFC alternative.
Properties
- Compound Formula: BrLi
- Molecular Weight: 86.85
- Appearance: White
- Melting Point: 552° C (1,026° F)
- Boiling Point: 1,265° C (2,309° F)
- Density: 3.46 g/cm3
- Solubility in H2O: 166.7 g/100 mL (20 °C)
- Exact Mass: 85.934342
- Monoisotopic Mass: 85.934342
Preparation
LiBr is soluble in water, alcohol, and ether. It is prepared by treating an aqueous suspension of lithium carbonate with hydrobromic acid or by reacting lithium hydroxide with bromine. It is prepared by the action of hydrobromic acid on lithium hydroxide. The salt forms several crystalline hydrates, unlike the other alkali metal bromides. Aqueous solutions of lithium bromide have usually low water vapor pressures. The anhydrous salt forms cubic crystals are similar to common salt (sodium chloride).
Lithium hydroxide and hydrobromic acid (aqueous solution of hydrogen bromide) will precipitate lithium bromide in the presence of water. Concentrated aqueous solutions of lithium bromide can dissolve significant quantities of polar organic substances such as cellulose.
LiOH + HBr → LiBr + H2O
Lithium bromide reacts with silver nitrate forms lithium nitrate and silver bromide. The chemical equation is given below.
LiBr + AgNO3 → LiNO3 + AgBr
Uses
- A 50–60% aqueous solution of lithium bromide is used in air-conditioning systems as desiccant.
- Used as a coolant in industrial air cooling systems working by absorptions.
- It is also used as a salt in absorption chilling along with water.
- Used in pharmaceuticals as a drying agent and for other industrial purposes.
- Solid LiBr is a useful reagent in organic synthesis.
- Used as a sedative and for the treatment of epilepsy.
- Used in the making of collodion dry plate emulsion due to its solubility in alcohol and ether.
Hazards
Lithium salts are psychoactive and somewhat corrosive. Heat is quickly generated when lithium bromide is dissolved into water because it has a negative enthalpy of solution.
Information Source: