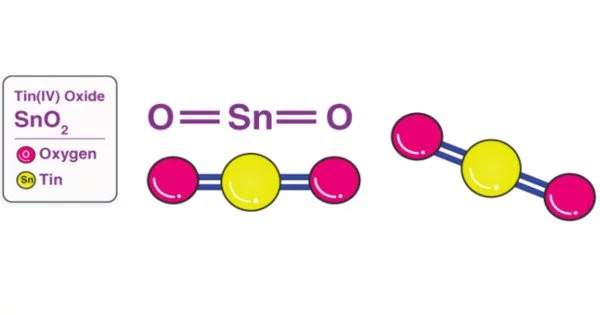
Tin(IV) oxide, or stannic oxide, is an inorganic compound with the formula SnO2. Cassiterite is the mineral form of SnO2, and it is the primary ore of tin. This tin oxide, also known by many other names, is an important material in tin chemistry. It is an amphoteric, colorless, diamagnetic solid.
Tin(IV) oxide occurs naturally as the mineral cassiterite, which is the main commercial source of tin. It is also used in a variety of applications, including as a polishing powder, as a catalyst in chemical reactions, as an opacifier in ceramics, and as a gas sensor in electronic devices.
Properties
- Chemical formula: O2Sn
- Molar mass: 150.708 g·mol−1
- Appearance: Yellowish or light grey powder
- Odor: Odorless
- Density: 6.95 g/cm3 (20 °C); 6.85 g/cm3 (24 °C)
- Melting point: 1,630 °C (2,970 °F; 1,900 K)
- Boiling point: 1,800–1,900 °C (3,270–3,450 °F; 2,070–2,170 K)
- Solubility in water: Insoluble
- Solubility: Soluble in hot concentrated alkalis, concentrated acids
- Insoluble in alcohol
Structure
The rutile structure is formed when tin(IV) oxide crystallizes. As a result, tin atoms have six coordinates and oxygen atoms have three coordinates. SnO2 is commonly thought of as an oxygen-deficient n-type semiconductor. Stannic acid has been described as hydrous forms of SnO2. Such materials appear to be hydrated SnO2 particles with a composition that reflects particle size.
Preparation
Tin(IV) oxide is found in nature. Tin(IV) oxide is created by burning tin metal in air. The annual output is in the range of 10 kilotons. In an industrial reverberatory furnace at 1200-1300 °C, SnO2 is reduced to metal with carbon.
Application
Tin(IV) oxide is commonly used as a pigment, as a polishing powder, and as a catalyst. It is also used in the manufacture of glass, ceramics, and semiconductors, and as a gas sensor in electronic devices.