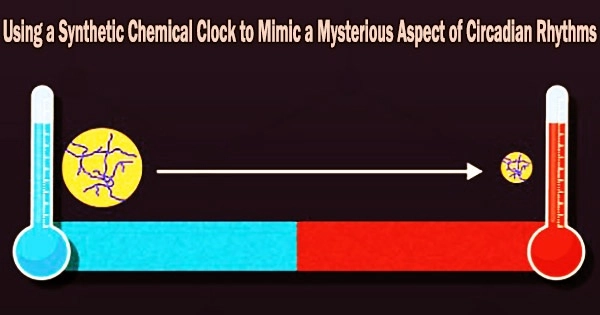
Circadian rhythms are regular internal oscillations that allow an organism to coordinate its physiological functions with its surroundings. These rhythms typically last for 24 hours and are controlled by molecular clocks that are located inside the body and react to external signals like light.
The oscillation period of circadian rhythms is not considerably influenced by temperature, despite the fact that the rate of the majority of metabolic reactions changes exponentially with temperature. Circadian rhythms are well understood in mammals, plants, and microbes. This demonstrates unequivocally the existence of a temperature-compensation system.
It’s interesting that some researchers have been successful in reproducing such temperature-invariant characteristics in a few oscillating chemical reactions. However, these reactions are frequently problematic and necessitate minute changes to the substances that are interacting.
But what if there was a simpler way to achieve temperature compensation in an oscillating chemical reaction?
Our study suggests that temperature compensation can be naturally self-sustainable through the output system of circadian machinery. This may explain why temperature compensation is a universal property of circadian rhythms seen in animals, plants, and bacteria, regardless of the molecular species involved.
Professor Yuhei Yamada
In a recent study published in Scientific Reports, a team of researchers including Assistant Professor Yuhei Yamada of Tokyo Institute of Technology (Tokyo Tech), Japan, came up with a clever idea for a temperature compensation mechanism using a reaction called the Belousov-Zhabotinsky (BZ) oscillating reaction.
The key to their approach lies in soft, temperature-responsive gels made from poly(N-isopropylacrylamide), or ‘PNIPAAm’ for short, in which the BZ reaction can occur. The polymeric strands that make up these gels can hold a specific amount of solvent. The amount of solvent present in these gels, however, reduces as temperature rises since these gels shrink as it does.
The researchers exploited this property of PNIPAAm gels by adding ruthenium (Ru) sites on its constituent polymers. The back-and-forth oxidation and reduction of ruthenium (Ru) ions plays a role in the periodic character of the specific BZ reaction that the researchers analyzed.
The relative concentrations of solvent and Ru, then, have an impact on the rate of this reaction. The relative concentration of Ru in the gels rises with temperature because shrinking PNIPAAm gels can hold less solvent.
The aforementioned effects come together to form a temperature-compensation mechanism that makes the time of the BZ reaction unaffected by changes in temperature, as the study team showed through experimental observations and a rigorous mathematical analysis.
“The prepared BZ gels exhibited temperature compensability just like the circadian rhythms observed in living organisms,” remarks Yamada.
Overall, this study shows a brand-new approach to temperature adjustment for periodic reaction-based artificial biological clocks.
Intriguingly, it’s even possible that similar temperature-compensation mechanisms using temperature-responsive soft bodies exist in biological systems in nature, as Yamada explains: “Our study suggests that temperature compensation can be naturally self-sustainable through the output system of circadian machinery. This may explain why temperature compensation is a universal property of circadian rhythms seen in animals, plants, and bacteria, regardless of the molecular species involved.”
Let us hope further studies can help us clarify the mysteries behind our internal clocks!