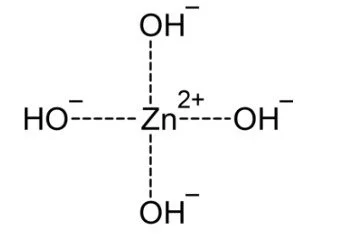
Sodium zincate is an inorganic compound with the chemical formula Na2ZnO2. It can refer to anionic zinc oxides or hydroxides depending on the circumstances. It is a white or off-white powder that is insoluble in water. It is an important intermediate in the production of zinc oxide and other zinc compounds. The exact formula is not always important in the applications of these materials, and aqueous zincate solutions are most likely mixtures.
Properties
- It is a white, crystalline solid that is soluble in water. It is often used as a precursor in the preparation of other zinc compounds, such as zinc oxide and zinc hydroxide.
- Melting point: The melting point of sodium zincate is 260°C.
- Solubility: Sodium zincate is soluble in water and forms a colorless solution.
- pH: Sodium zincate has a basic pH, with a pH of around 10.
- Density: The density of sodium zincate is approximately 2.26 g/cm3.
- Reactivity: Sodium zincate is reactive with acids, producing zinc salts and regenerating sodium hydroxide. It is also used as a strong alkaline agent in chemical reactions.
- Stability: Sodium zincate is relatively stable under normal conditions, but it can decompose under high temperatures.
Preparation
Sodium zincate can be prepared by reacting zinc oxide with sodium hydroxide solution. The resulting solution contains sodium zincate and water. The sodium zincate can be precipitated from the solution by adding a salt that forms an insoluble compound with sodium, such as sodium chloride or sodium sulfate. The resulting solid can be further purified by washing with water.
Solutions of sodium zincate may be prepared by dissolving zinc, zinc hydroxide, or zinc oxide in an aqueous solution of sodium hydroxide. Simplified equations for these complex processes are:
ZnO + H2O + 2 NaOH → Na2Zn(OH)4
Zn + 2 H2O + 2 NaOH → Na2Zn(OH)4 + H2
From such solutions, one can crystallize salts of containing the anions Zn(OH)42−, Zn2(OH)62−, and Zn(OH)64−. Na2Zn(OH)4 consists of tetrahedral zincate ion and octahedral sodium cations.
The salt Sr2Zn(OH)6 features zinc in an octahedral coordination sphere.
Applications
Sodium zincate is used in a variety of applications, including as a catalyst in organic synthesis, as a component in electroplating solutions, and as a starting material for the production of other zinc compounds. It is also used in the production of pigments, ceramics, and glass.
In addition to its industrial applications, sodium zincate has also been studied for its potential use in wastewater treatment, as it can help remove heavy metals from contaminated water. However, further research is needed to determine its effectiveness and safety in this application.