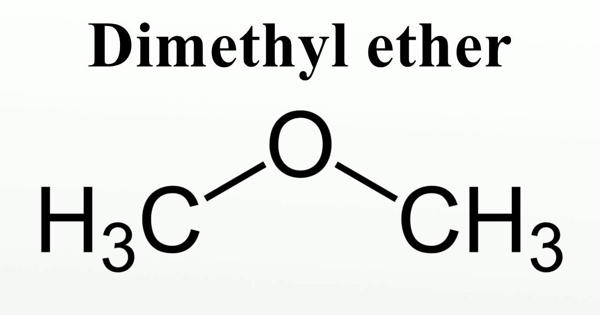
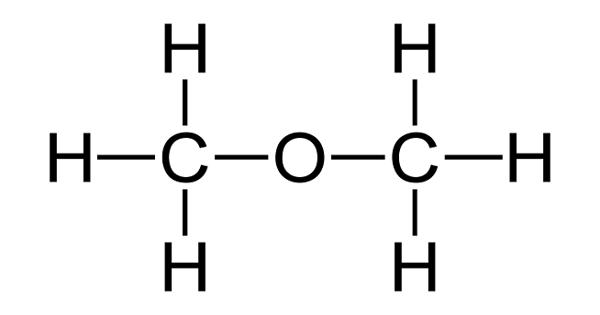
Dimethyl ether (DME) is a synthetically produced alternative to diesel for use in specially designed compression ignition diesel engines. It is also known as methoxymethane, is the organic compound with the formula CH3OCH3, simplified to C2H6O. It is used extensively in the chemical industry and as an aerosol propellant. The simplest ether, it is a colorless gas that is a useful precursor to other organic compounds and an aerosol propellant that is currently being demonstrated for use in a variety of fuel applications. It is a colorless volatile poisonous liquid compound used as a solvent, fuel, aerosol, propellant, and refrigerant. It is an isomer of ethanol.
Production
Although dimethyl ether can be produced from biomass, methanol, and fossil fuels, the likely feedstock of choice for large-scale DME production in the United States is natural gas. Approximately 50,000 tons were produced in 1985 in Western Europe by dehydration of methanol:
2 CH3OH → (CH3)2O + H2O
DME can be produced directly from synthesis gas produced from natural gas, coal, or biomass. The required methanol is obtained from synthesis gas (syngas). Other possible improvements call for a dual catalyst system that permits both methanol synthesis and dehydration in the same process unit, with no methanol isolation and purification. Both the one-step and two-step processes above are commercially available. It can also be produced indirectly from methanol via a dehydration reaction.
Applications
Dimethyl ether has several fuel properties that make it attractive for use in diesel engines. It has a very high cetane number, which is a measure of the fuel’s ignitibility in compression ignition engines. The largest use of dimethyl ether is as the feedstock for the production of the methylating agent, dimethyl sulfate, which entails its reaction with sulfur trioxide:
CH3OCH3 + SO3 → (CH3)2SO4
Dimethyl ether can also be converted into acetic acid using carbonylation technology related to the Monsanto acetic acid process:
(CH3)2O + 2 CO + H2O → 2 CH3CO2H
Laboratory reagent and solvent
Dimethyl ether is a low-temperature solvent and extraction agent, applicable to specialised laboratory procedures. Its usefulness is limited by its low boiling point (−23 °C (−9 °F)), but the same property facilitates its removal from reaction mixtures.
Niche applications
A mixture of dimethyl ether and propane is used in some over-the-counter “freeze spray” products to treat warts, by freezing them. In this role, it has supplanted halocarbon compounds (Freon).
It is a synthetic fuel, gaseous at ambient conditions, which can be liquefied at moderate pressure allowing it to be handled as a liquid in a similar way to LPG. Dimethyl ether is also used as a propellant in aerosol products. Such products include hair spray, bug spray and some aerosol glue products.
Information Source: