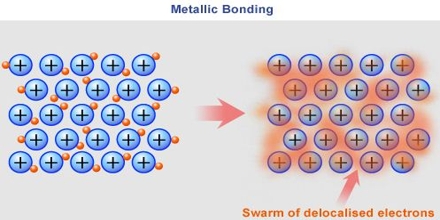
Basic objective of this lecture is to describe on Bonding in Metals. Metals are made up of closely packed cations, they are not neutral atoms and valence e- can move. Free floating valence e- are attracted to (+) metal ions. Metal atoms are arranged in very compact & orderly patterns. Here explain bonding in metals with chemical reactions and pictures. Some examples of metals are: Sterling Silver, Bronze, Steel, Titanium etc.
Bonding in Metals