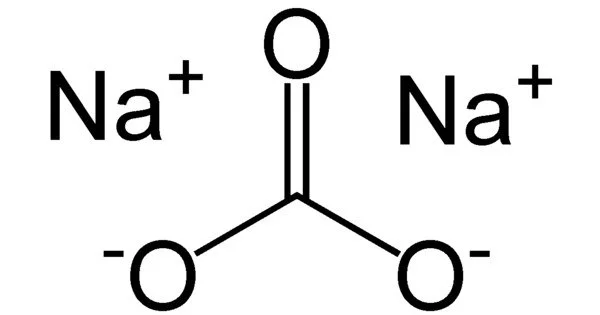
Sodium carbonate (also known as washing soda, soda ash, and soda crystals) and its various hydrates are inorganic compounds with the formula Na2CO3. All forms are white, odorless, water-soluble salts that produce alkaline water solutions.
The white, odorless solid sodium carbonate has a high melting point. It dissolves in water and produces alkaline solutions. The molecular weight of the compound is 105.99 g/mol. It occurs naturally in mineral deposits known as trona or nahcolite. These minerals are mined and processed in order to produce sodium carbonate.
Properties
- Physical state: It exists as a solid at room temperature and pressure. It crystallizes as a decahydrate (Na2CO3·10H2O), known as soda ash or soda crystals, which is a white, odorless powder. The anhydrous form is a hygroscopic powder.
- Solubility: It is highly soluble in water. It readily dissolves in water to form an alkaline solution. The solubility of sodium carbonate decreases with decreasing temperature.
- Alkalinity: It is a strong alkali and has a high pH value. When dissolved in water, it dissociates into sodium ions (Na+) and carbonate ions (CO32-), which contribute to the alkalinity of the solution. This alkaline property makes it useful in various industrial and household applications.
- Chemical reactivity: It is a versatile compound and reacts with various substances. It reacts with acids to form salt and water through a neutralization reaction. It also reacts with certain metals, such as aluminum and zinc, producing hydrogen gas. Sodium carbonate can also undergo thermal decomposition at high temperatures.

Production
Sodium carbonate can be produced using a variety of methods, including the Solvay process, the Leblanc process (now obsolete), and natural deposit mining.
It was traditionally extracted from the ashes of plants grown in sodium-rich soils. Because the ashes of these sodium-rich plants differed noticeably from those of wood (which was once used to produce potash), sodium carbonate was dubbed “soda ash.” It is made in large quantities by the Solvay process from sodium chloride and limestone, as well as by carbonating sodium hydroxide produced by the Chlor-alkali process.
Uses
In the industry, sodium carbonate is widely used as a pH regulator, a cleaning agent, and a water softener. It’s also used in the production of glass, soaps, detergents, and paper.
In water treatment, it is used to adjust the pH and neutralize acidic conditions. Because of its alkaline properties, it can be used to clean laundry or household surfaces. It’s also used as a food additive (E500) in some products like baking soda.
Safety Precautions
Although sodium carbonate is generally considered safe to handle and use, it can cause skin, eye, and respiratory system irritation. When working with this chemical, proper safety precautions and guidelines should be followed.