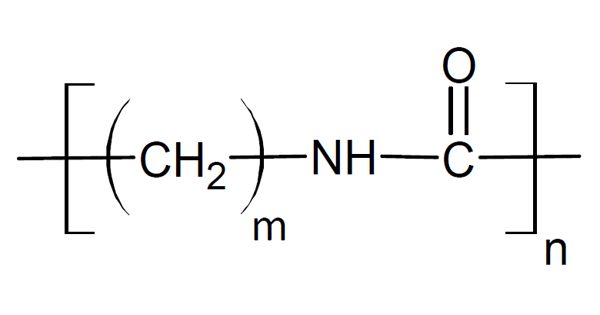
The synthetic sulfate salt of hydrazine, a derivative of ammonia, is hydrazine sulfate, more accurately called hydrazinium hydrogensulfate. It is the anion bisulfate (hydrogensulfate), with the formula (N2H+5) (HSO−4) or N2H6SO4. At room temperature, it is a white, water-soluble solid. Hydrazine sulfate is a salt formed by hydrazine and sulfuric acid, a colorless scaly crystal or rhombic pure product; the molecular weight is 130.12. The melting point is 254°C; it will decompose as heating continues; the relative density is 1.37. It is mildly soluble in water, soluble in hot water (at 20°C 2.87, at 25°C 3.41, at 30°C 3.89, at 40°C 4.16, at 50°C 7.0, at 60°C 9.07, at 80°C 14.4), acidic, alcohol and ether insoluble in aqueous solution. In chemical laboratories and in the chemical industry, hydrazine sulfate has many applications, including analytical chemistry and organic compound synthesis. Pure hydrazine is commonly favored in such applications because it is not reactive and less vulnerable to atmospheric storage oxidation.
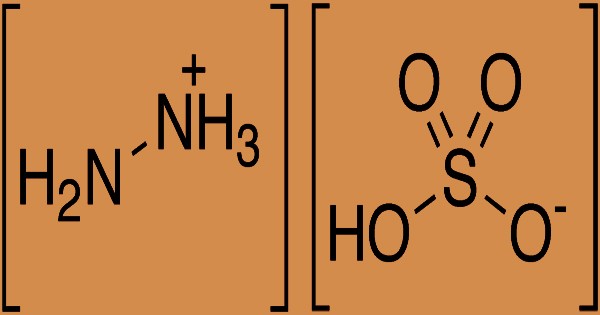
The enzyme phosphoenol pyruvate carboxykinase is inhibited by hydrazine, thereby preventing gluconeogenesis. This agent has been reported to minimize cancer patients’ excessive energy needs and cachexia. By treating an aqueous hydrazine solution (N2H4) with sulfuric acid (H2SO4), the compound can be prepared. In the air, hydrazine sulfate is very stable. It is prone to alkali and oxidizing agents and is incompatible with oxidizing agents and bases. There’s a strong decline. LD50601mg/kg oral rodent, toxic, carcinogenic. For the determination of the weight of nickel, cobalt, cadmium and the purification of rare metals, the isolation of tellurium and polonium, as well as the precipitation of chlorate, hypochlorite, and carboxyl compounds, the main function of hydrazine sulphate is to be used. It is also used as a reduction agent, insecticides, and sterilizing agents for the manufacture of isoniazid, nitrofurazone, 100 Health hydrazine, anhydrous hydrazine, etc.
In addition to its general use as a safe source of hydrazine, the compound is used as a catalyst to produce acetate fibers, to analyze and synthesize minerals, and to test metals for arsenic. Hydrazine sulfate is also listed as a possible human carcinogen and is a weak monoamine oxidase inhibitor (MAO). It can be used as an analytical reagent and a reduction agent, and can also be used for rare metal purification. As an alternative medical treatment for appetite reduction (anorexia) and rapid weight loss (cachexia), which are also associated with cancer, hydrazine sulfate has been used.
For the gravimetric calculation of nickel, cobalt and cadmium; for the refining of rare metals; for the antioxidant soldering flux for light metals; for the reduction agent in mineral and slag analysis; for the separation of polonium from tellurium; for blood tests; for the removal of fungi and molds; for the preparation of hydrazine hydrate. Hydrazine sulfate has never been approved in the United States as safe and effective in treating any medical condition, although it has been sold as a dietary supplement.
Hydrazine sulfate in a two-step phase can be synthesized from aqueous ammonia and sodium hypochlorite solution. In the first step, the aqueous ammonia solution is boiled with a standard sodium hypochlorite solution in the presence of a hydrazine-producing 10% gelatin solution. The hydrazine solution is ice-cooled in the second step, followed by the gradual addition of concentrated sulfuric acid. An analysis of clinical literature concluded that it has never been proven that hydrazine sulfate acts as an anticancer agent; patients do not undergo cancer remissions or regressions, and patients do not live longer than patients who have not been treated. The compound has been described by some clinical reviews of alternative cancer treatments as a “disproved and ineffective treatment for cancer”. Based on ample evidence of carcinogenicity from experiments in laboratory animals, hydrazine and hydrazine sulfate are fairly anticipated to be human carcinogens. Sulfate hydrazine is toxic and potentially carcinogenic. The short-term side effects reported in different clinical trials, however, are relatively mild: moderate nausea and vomiting, dizziness and arousal, polyneuritis (inflammation of the nerves) and fine muscle control difficulties (such as writing). However, in rare cases, more extreme, even fatal side effects have been reported: one patient developed fatal liver and kidney failure, and another patient developed significant neurotoxicity symptoms.
Information Sources: