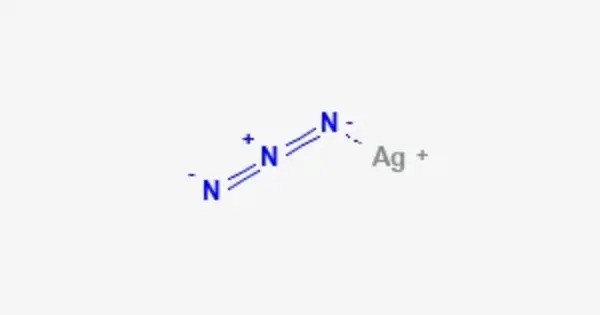
Silver azide is the chemical compound with the formula AgN3. It is a silver(I) salt of hydrazoic acid. It forms a colorless crystals. Like most azides, it is a primary explosive. It is a white, crystalline substance that is highly sensitive to shock, heat, and friction, which makes it a dangerous explosive material. It is used in some specialized applications, such as in the production of primers for explosives or in organic synthesis.
Properties
It is typically a white, crystalline solid, although it may appear somewhat yellowish due to its sensitivity to light. It is highly sensitive to heat, shock, and friction. It can decompose explosively, especially when subjected to mechanical shock or strong heating. This makes it hazardous to handle. It is not very soluble in water but can dissolve in ammonia or other solvents under certain conditions.
- Chemical formula: AgN3
- Molar mass: 149.888 g/mol
- Appearance: colorless crystals
- Density: 4.42 g/cm3
- Melting point: 250 °C (482 °F; 523 K) explosive
- Boiling point: decomposes
- Solubility in other solvents: 2.0×10−8 g/L
- Crystal structure: Orthorhombic oI16
Structure and chemistry
Silver azide can be prepared by treating an aqueous solution of silver nitrate with sodium azide. The silver azide precipitates as a white solid, leaving sodium nitrate in solution.
AgNO3(aq) + NaN3(aq) → AgN3(s) + NaNO3(aq)
X-ray crystallography shows that AgN3 is a coordination polymer with square planar Ag+ coordinated by four azide ligands. Correspondingly, each end of each azide ligand is connected to a pair of Ag+ centers. The structure consists of two-dimensional AgN3 layers stacked one on top of the other, with weaker Ag–N bonds between layers.
Occurrences
Synthesis: Silver azide is typically synthesized in a laboratory by reacting silver nitrate (AgNO₃) with sodium azide (NaN₃) in solution:
AgNO₃ (aq)+NaN₃ (aq)→AgN₃ (s)+NaNO₃ (aq)
Not naturally occurring: Silver azide does not occur naturally in significant quantities. Its use is mainly in chemical research and sometimes in specialty applications.
Due to its explosive nature, silver azide is often handled with great care in controlled environments, and its use is primarily limited to scientific or industrial contexts where its reactivity can be studied or exploited safely.