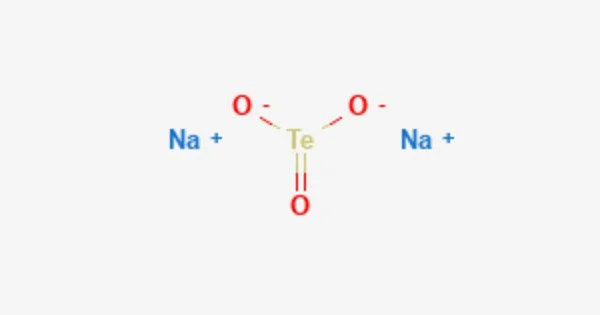
Sodium telluride is an inorganic compound with the chemical formula Na2Te. It is a dark gray or black crystalline solid that is highly reactive and soluble in water. Sodium telluride is composed of sodium cations (Na+) and telluride anions (Te2-).
It is a chemical compound with the formula Na2Te. This salt is the conjugate base of the thermally unstable acid hydrogen telluride, but it is typically prepared by reducing tellurium with sodium. Because it is extremely sensitive to air, Na2Te is a difficult material to work with. Air oxidizes it first to polytellurides, which have the formula Na2Tex (x > 1), and then to Te metal. Because of the effects of air oxidation, samples of Na2Te, which is colorless when absolutely pure, generally appear purple or dark gray.
Properties
- Appearance: It is a grayish-white solid.
- Solubility: It is soluble in water and insoluble in organic solvents.
- Odor: It is odorless.
- Melting and boiling point: It has a melting point of 636°C and a boiling point of 1310°C.
- Density: The density is 3.01 g/cm³.
- Conductivity: It is an ionic compound and conducts electricity when dissolved in water.
Application
Sodium telluride is commonly used as a reducing agent in various chemical reactions, particularly in the synthesis of organotellurium compounds. It is also used in the production of semiconductors and as a catalyst in the manufacturing of polymers. However, sodium telluride is highly toxic and should be handled with extreme caution. Its use and disposal are strictly regulated due to environmental and health concerns.
Sodium telluride is used as a source of tellurium for the production of semiconductors, in the manufacture of glass, and as a reagent in organic synthesis. It can also be used as a reducing agent in chemical reactions.
Safety
Sodium telluride is highly toxic and should be handled with care. It can release toxic fumes of tellurium and sodium oxides when heated or exposed to acids. It can also cause severe irritation to the eyes, skin, and respiratory system, so protective gear such as gloves and a respirator should be worn when working with it.