A transparent colorless to yellow volatile liquid, nickel carbonyl (IUPAC name: tetracarbonylnickel), is flammable and burns with a yellow flame. It is the organonickel compound with the formula Ni(CO)4; the principal carbonyl of nickel is this colorless oil. Soluble in alcohol, benzene, chloroform, acetone, ethanol, carbon tetrachloride, and nitric acid, it is denser than water and is insoluble in water. For the processing of a very high-purity nickel and a reagent in organometallic chemistry, it is an intermediate in the Mond process, although the Mond process has fallen out of common use due to the health hazards of dealing with the compound.

(Nickel tetracarbonyl)
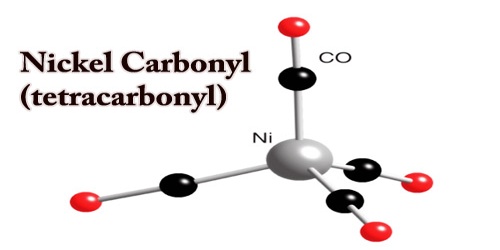
Nickel carbonyl is used in nickel coating steel and other metals in industries and in the processing of very pure nickel. Because of its extremely high toxicity, compounded with high volatility and fast skin absorption it is one of the most harmful substances yet found in nickel chemistry. Nickel carbonyl becomes peroxidized as a solid deposition by sunlight and decomposes to ignite. The oxidation state for nickel is assigned as zero in Nickel tetracarbonyl. The formula is consistent with the 18-electron law. The molecule is tetrahedral and has four ligands of carbonyl (carbon monoxide). Electron diffraction studies have been performed on this molecule, and the Ni–C, and C–O distances have been calculated to be 1.838(2) and 1.141(2) angstroms respectively.
A team led by scientist and industrialist Ludwig Mond first found nickel carbonyl while working in a lab in the converted stables of his home in north London. Mond was already popular and wealthy at the time of the discovery, having supervised the large-scale commercialization of the Solvay method used to create sodium carbonate. It is the most significant zero-valent nickel compound and is used industrially for the production of high-purity nickel powder and pellets and for the production of nickel coatings on steel. At 323 K (50 °C; 122 °F), carbon monoxide is passed over impure nickel. The optimal rate occurs at 130 °C.
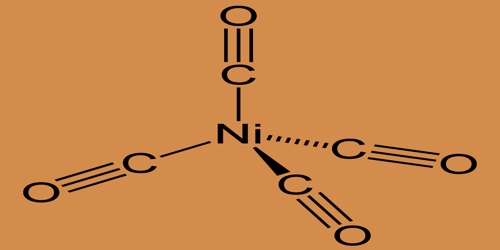
Structure of Nickel Carbonyl (tetracarbonyl)
Nickel tetracarbonyl (Ni(CO)4) is not readily commercially available. It is conveniently generated by carbonylation of commercially available bis(cyclooctadiene)nickel(0) in the laboratory. It can also be prepared by reducing nickel sulfate ammonia solutions with sodium dithionite in the CO atmosphere. It is used as a catalyst for carboxylation, coupling, and cyclization reactions in the manufacture of nickel powder and nickel-coated metals. It can form from interaction with nickel through carbon monoxide.
Carbonyl nickel can be oxidized. Chlorine oxidizes the carbonyl nickel into NiCl2, which releases CO steam. Other halogens behave similarly. A convenient method for precipitating the nickel component of the toxic compound is given by this reaction. It is obtained by moving carbon monoxide at 50 to 100 ° C over finely divided nickel. The hazards of Ni(CO)4 are far greater than that implied by its CO content, reflecting the consequences of the nickel if released within the body. In several commercial processes, the reaction is administrated at a temperature of 200°C under 400 atm carbon monoxide gas pressures for obtaining high yield of nickel tetracarbonyl and also to forestall thermal dissociation.
Because of its high volatility, nickel carbonyl can be lethal if ingested through the skin, or more likely inhaled. The LC50 has been measured at 3 ppm for a 30-minute exposure, and the concentration that is instantly lethal to humans would be 30 ppm. While skin contact with a dilute solution can cause dermatitis and itching, a burn can be created from a concentrated solution of pure liquid. Absorption of the liquid through the skin may lead to death. Poisoning with nickel carbonyl is characterized by a two-stage disease. The first consists of headaches and hurting lasting some hours, usually followed by a brief remission. The second phase may be a chemical pneumonitis which starts after typically 16 hours with symptoms of cough, breathlessness, and extreme fatigue. After four days, these attain the greatest severity, likely resulting in death from cardiorespiratory or acute kidney injury.
Working with nickel carbonyl should be performed in a fume hood to avoid damage by inhalation and splash goggles, and impermeable gloves should be worn at all times to avoid contact with the eyes and skin. Carbonyl nickel can only be used in areas free from sources of ignition. In areas isolated from oxidizers, nickel carbonyl containers should be placed in secondary containers in the dark. The carcinogenicity of Ni(CO)4 is a matter of debate but is presumed to be significant. In the United States, it is listed as an extremely dangerous drug, as described in Section 302 of the U.S. Emergency Planning and the Community Right-to-Know Act (42 U.S.C. 11002), and facilities that manufacture, store, or use it in large amounts are subject to stringent reporting standards.
Information Sources: