Potassium chromate, commonly known as tarapacaite, is a yellowish, crystalline, inorganic compound that emits toxic chromium fumes upon heating. The formula for potassium chromate is K2CrO4. It is an inorganic substance that crystallizes at room temperature as a yellow orthorhombic or hexagonal structure. The melting point is 968 °C, and the relative density is 2.732.
The potassium salt of the chromate anion is represented by this yellow solid. It is a typical laboratory substance, whereas sodium chromate plays a significant role in the industry. Potassium chromate is a potent oxidizer and very corrosive substance. This material is employed in the production of dyes as well as textile dying procedures. The primary risk is the environmental threat; prompt action should be taken to stop its proliferation inside the ecosystem.
It can dissolve in water to create an alkaline chromate ion hydrolysis solution and is hazardous. Additionally, alcohol and ether cannot dissolve it. The potassium chromate solution will change from yellow to orange, which is the color of dichromate when acid is added. Oxidizing agents are chemicals that, through a chemical reaction, oxidize other compounds. An oxidizing agent causes a substance to lose electrons during the oxidation process.
Potassium chromate is a chemical that has been associated to an increased risk of developing cancer and sinonasal cavity lung cancer in humans. It decomposes when heated and releases poisonous fumes of potassium oxide. It is known to exist in two crystalline forms, both of which resemble the equivalent potassium sulfate.
The typical form of β-K2CrO4 is orthorhombic, however, beyond 66 °C, it transforms into a -form. Despite the chromate ion adopting the conventional tetrahedral geometry, these structures are complicated. Two positively charged potassium cations (K+ ions) and one chromate anion make up potassium chromate (CrO42- ion).
Below is a diagram illustrating potassium chromate’s structure. Two double and two single covalent bonds are formed between the core chromate ion and oxygen. The image’s dotted lines show the various places where single and double bonds between chromium and oxygen can form. The chromate ion is then ionically bonded to 2 potassium ions.
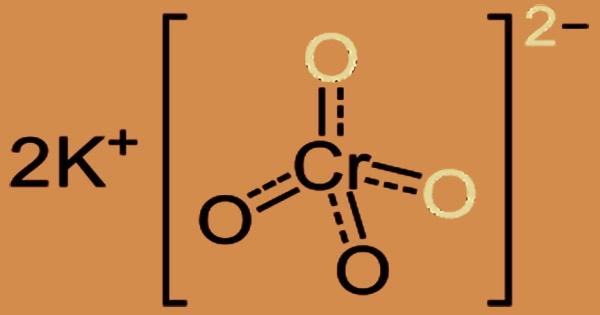
One chromium atom and four oxygen atoms combine to form the chromate anion. The center chromium atom in this polyatomic ion is singly bonded to two oxygen atoms and doubly bound to two oxygen atoms. Potassium chromate has oxidative characteristics and can produce Cr(OH)4- (specifically CrO2-) when it reacts with a reducing agent in an alkaline media.
When various solutions containing barium, lead, and silver ions are added to a potassium chromate solution, the resulting insoluble chromates are formed: barium chromate BaCrO4 (yellow), lead chromate pbCrO4 (yellow), and silver chromate Ag2CrO4 (Brick red).
While potassium chromate mostly harms the nose, throat, and lungs, it can also harm the digestive system, liver, kidneys, and immune system. These harms include ulcers, shortness of breath, bronchitis, pneumonia, and asthma. This chemical is a known human carcinogen that has been linked to an elevated risk of lung and sinonasal cavity cancer.
The mineral form of potassium chromate is called tarapacaite. In 1878, Tarapaca, Chile, saw the discovery of this brilliant yellow mineral. The mineral is extremely uncommon and is only found in a few places. The potassium chromate indicator method also referred to as the Moore (Mohr) method, is a precipitation titration technique that use silver nitrate (AgNO3) as the standard solution and potassium chromate (K2CrO4) as the indicator.
By processing potassium dichromate and potassium hydroxide, potassium chromate can be created. Chromium is most frequently found in the oxidation states +2, +3, and +6. Its atomic number is 24 and its electronic configuration is 3d54s1. When potassium dichromate and potassium hydroxide combine, potassium chromate is created.
K2Cr2O7 + KOH → K2CrO4+H2O
When potassium dichromate and potassium carbonate combine, potassium chromate is also produced.
K2Cr2O7 + K2CO3 → K2CrO4 + CO2
A potassium salt called potassium chromate is made up of ions of potassium and chromate in a 2:1 ratio. It functions as an oxidizing agent and a carcinogen. Potassium and sodium dichromates behave quite similarly in solutions. Lead(II) chromate, an orange-yellow precipitate, is produced when it is combined with lead(II) nitrate.
The only Cl- or Br-ions that can be directly titrated using the potassium chromate indicator method are their totals when coexisting. Since they are too easily absorbed by sedimentation and the endpoint is ambiguous, this approach is not appropriate for determining iodide ions (I-) or thiocyanate ions (SCN-).
The reverse titration method, which involves adding an excessive amount of NaCl standard solution to the test solution and then using AgNO3 standard solution to titrate excess Cl-ions, can be used to determine Ag+ even though this approach is not ideal for titrating Ag+ with Cl-. leaching, treating the cinder with a hot potassium sulfate solution and roasting powdered chromite with potash and limestone.
When an anhydrous salt is required, potassium salt is typically employed in laboratories rather than the more affordable sodium salt. For example, it is employed as a colorimetric test for silver ions in qualitative inorganic analysis. As potassium chromate glows red in the presence of excess silver ions, it is also employed as an indicator in precipitation titrations with silver nitrate and sodium chloride (they can be used as standard and titrant for each other).
The principal use of the potassium chromate indicator method is the detection of Cl-ions in extremely diluted solutions, such as in drinking water and industrial product contaminants. It is mostly employed in the production of chemical reagents and pigments, but it is also used for ink, paint, enamel, metal corrosion, and other things.