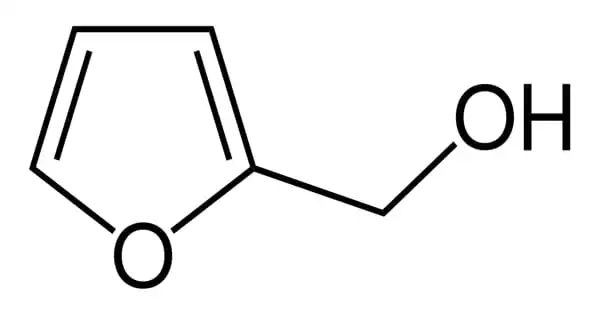
Furfuryl alcohol is an organic compound that contains a furan group that has been substituted with a hydroxymethyl group. It has the appearance of a clear, colorless liquid. Although it is a colorless liquid, aged samples appear amber. It is a furan with a 2-position hydroxymethyl substituent. It has a faint burning odor and a bitter taste. It plays a role in the Maillard reaction. It is miscible with water but unstable in it. It is a type of primary alcohol and a furan. It is soluble in a variety of organic solvents.
Properties
Its flash point is 167°F and oiling point 171°F. It is denser than water. Contact may irritate skin, eyes and mucous membranes. It may be toxic by ingestion and skin contact and moderately toxic by inhalation. It is an efficient trapping agent for singlet oxygen determination in natural waters.
- Melting point: -29 °C (lit.)
- Boiling point: 170 °C (lit.)
- Density: 1.135 g/mL at 25 °C (lit.)
- Vapor density: 3.4 (vs air)
- Vapor pressure: 0.5 mm Hg ( 20 °C)
- Form: Liquid
- Color: Clear yellow
- Odor: Mildly irritating

Synthesis
Furfuryl alcohol is produced industrially through the hydrogenation of furfural, which is typically produced from waste bio-mass such as corncobs or sugar cane bagasse. As a result, furfuryl alcohol may be classified as a green chemical. Using solid acid catalysts, one-pot systems for producing furfuryl alcohol directly from xylose have been investigated.
Reactions
Furfuryl alcohol was used as a carbon source in the synthesis of titanium carbide nanofibers/nanoribbons, which are useful in the preparation of carbide-derived carbon (CDC) materials.
It is subjected to a variety of reactions, including Diels-Alder additions to electrophilic alkenes and alkynes. 1,5-bis(hydroxymethyl)furan is produced by hydroxymethylation. Levulinic acid is produced by hydrolysis. Furfuryl alcohol can be polymerized into a resin by treating it with acids, heat, and/or catalysts (furfuryl alcohol). Furfuryl alcohol can be hydrogenated to produce hydroxymethyl derivatives of tetrahydrofuran and 1,5-pentanediol. The etherification of furfuryl alcohol with alkyl or aryl halide (e.g., benzyl chloride) in a liquid-liquid-liquid triphase system using a phase transfer catalyst was also reported. Furfuryl alcohol is converted to a dihydropyran in the Achmatowicz reaction, also known as the Achmatowicz rearrangement.
Preparation
Typically prepared from furfural obtained through the processing of corncobs; oil obtained through the steam distillation of roasted coffee bean meal contains 50% furfuryl alcohol; industrially prepared through the catalytic reduction of furfural using nickel and Cu-CrO catalysts.
Applications
Furfuryl alcohol is primarily used as a monomer in the synthesis of furan resins. Thermoset polymer matrix composites, cements, adhesives, coatings, and casting/foundry resins all use these polymers.
Furfuryl alcohol has been used in rocketry as a fuel that ignites hypergolically (immediately and energetically in contact) with white fuming nitric acid or red fuming nitric acid oxidizer. Using hypergolics eliminates the need for an igniter.
Furfuryl alcohol, due to its low molecular weight, can impregnate the cells of wood, where it can be polymerized and bonded with the wood via heat, radiation, and/or catalysts or additional reactants. The treated wood has improved moisture-dimensional stability, hardness, and decay and insect resistance; catalysts can include zinc chloride, citric acid, formic acid, and borates.
It can also be used as follows:
- As a starting material for the production of ethyl levulinate, a biofuel derived from lignocellulosic residues.
- To prepare colloidal microporous carbon spheres, applicable as adsorbents and catalyst supports.
- As a starting material to prepare cyclopentanone through aqueous phase hydrogenation reaction using metal catalysts.
Safety
Furfuryl alcohol’s median lethal dose ranges from 160 to 400 mg/kg (mouse or rabbit, oral). Poisoning can occur through ingestion, skin contact, or subcutaneous contact. Inhalation and intraperitoneal routes are moderately toxic. Data on mutations was reported. An irritant to the eyes. When exposed to heat or flame, it becomes flammable and can react with oxidizing materials. When exposed to heat or flame, there is a moderate risk of explosion.