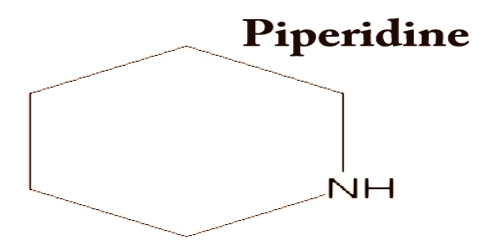
Piperidine is found in barley, and it is an organic compound with the molecular formula (CH2)5NH. It is a clear, colorless liquid; Pepper, ammonia or amine odor. This heterocyclic amine consists of a six-membered ring containing five methylene bridges (–CH2–) and one amine bridge (–NH–). Piperidine (Piper nigrum) is found in black pepper. The name comes from the name of the genus Piper; this is the Latin word for pepper.
Piperidine is a strong base (pKb = 2.88) that reacts vigorously with oxidizing materials, is easily ignited, and forms explosive vapor concentrations at room temperature. While piperidine is a popular organic compound in many pharmaceuticals and alkaloids, such as natural-occurring solenopsins, it is best known as a representative structural element. When heated to decomposition it gives off toxic fumes of NOx, and it behaves like an aliphatic secondary amine and can form complexes with salts of heavy metals
Piperidine is a commonly used building block and chemical reagent for organic compound synthesis, including pharmaceutics. It has a thick, spicy, floral, animal smell and a taste of burning peppery. Industrially, piperidine is produced by the hydrogenation of pyridine, usually over a molybdenum disulfide catalyst:
C5H5N + 3 H2 → C5H10NH
Using sodium in ethanol, pyridine can also be reduced to piperidine by a modified reduction of Birch. It is a commonly used secondary amine and is also commonly used for converting ketones into enamins.
Piperidine actually occurs at low levels in a variety of food products, including baked ham (0.2 p.p.m.), milk (0.11 p.p.m.) coffee (1 p.p.m. dry) and canned fish; it is also found in black pepper, hemp, hemlock, and tobacco. Even piperidine is used as a solvent and as a base. Similar to cyclohexane, piperidine favors a chair conformation. It has a function as a reagent, a protic solvent, a base, a catalyst, a metabolite of plants, a metabolite of humans, and a non-polar solvent. Piperidine is a natural constituent of skin, human urine, brain, and cerebrospinal fluid; Humans excrete about 3-20 mg/d in the urine.
An important industrial use of piperidine is for the manufacture of tetrasulfide dipiperidinyl dithiuram, which is used as an accelerator of rubber sulfur vulcanisation. Piperidine may also be obtained by heating piperidine with alcoholic KOH or by the cyclization of 1,5-diaminopentane hydrochloride. Will float on water; Flashpoint 37°F; Melting point -15.8°F (-9°C); Boiling point 222.8°F (106°C). Commercial piperidine comes in two classes, pure at 95 and 98 percent. Irritate the skin and eyes severely; may be poisonous through absorption and inhalation; may be highly flammable and miscible in water.
Piperidine is absorbed readily through the gastrointestinal tract, skin, and lungs. It is also widely used in reactions to chemical degradation, such as DNA sequencing in the cleavage of particular modified nucleotides. Piperidine and most of its derivatives are easily produced by hydrogenating the corresponding pyridine derivatives over nickel, palladium, or ruthenium catalysts at elevated temperature and pressure. It is a highly flammable liquid, and vapor may form an explosive mixture with air (at room temperature).
Information Sources: