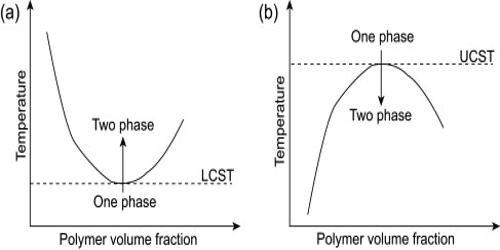
The lower critical temperature varies greatly, depending on the `normal’ temperature regime to which the organism is adapted. The lower critical solution temperature (LCST) or lower consolute temperature is the critical temperature below which the components of a mixture are miscible for all compositions. Critical solution temperature is the temperature at which complete miscibility is reached as the temperature is raised or in some cases lowered —used of two liquids that are partially miscible under ordinary conditions. The word lower indicates that the LCST is a lower bound to a temperature interval of partial miscibility or miscibility for certain compositions only. The temperature-induced phase transition may go hand in hand with other stimuli, such as a change in the pH of the solution, as is the case with poly (dimethylaminoethyl methacrylate) and polymers with similar structures.
The phase behavior of polymer solutions is an important property involved in the development and design of most polymer-related processes. Thermoresponsive polymers that undergo reversible phase transition by responding to an environmental temperature change, in particular polymers showing lower critical solution temperature, are frequently used as smart materials that have found increasing applications. Partially miscible polymer solutions often exhibit two solubility boundaries, the upper critical solution temperature (UCST) and the lower critical solution temperature (LCST), which both depend on the molar mass and the pressure. At temperatures below LCST, the system is completely miscible in all proportions, whereas above LCST partial liquid miscibility occurs. Recently, there has been a rapid growth in interest in LCST polymers and many new research groups are entering the field from a wide range of application areas.
In the phase diagram of the mixture components, the LCST is the shared minimum of the concave up spinodal and binodal (or coexistence) curves. It is in general pressure-dependent, increasing as a function of increased pressure. The phase behavior at high temperatures of non‐polar polymer solutions is discussed in a qualitative way in terms of the free volume theory of liquids developed by Prigogine, Patterson, and Flory.
The Lower Critical Solution Temperature (LCST) of polyethylene (PE), polypropylene (PP), and ethylene‐propylene block and random copolymers have been measured in heptanes. For small molecules, the existence of an LCST is much less common than the existence of an upper critical solution temperature (UCST), but some cases do exist. For example, the system triethylamine-water has an LCST of 19 °C, so that these two substances are miscible in all proportions below 19 °C but not at higher temperatures. In addition, the values of the LCST increase as the length of the alkyl chain in the imidazolium cation increases. The nicotine-water system has an LCST of 61 °C, and also a UCST of 210 °C at pressures high enough for liquid water to exist at that temperature. By using ionic liquid blends as solvents, the LCSTs can be tuned by varying the mixing ratio of two ionic liquids. The components are therefore miscible in all proportions below 61 °C and above 210 °C (at high pressure), and partially miscible in the interval from 61 to 210 °C.