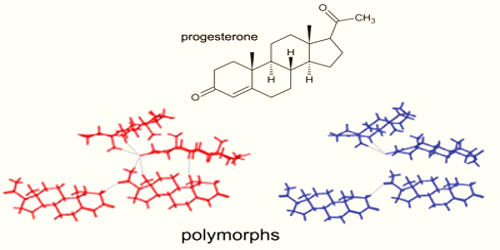
Polymorphism is a common phenomenon of crystalline materials. In materials science, polymorphism is the ability of a solid material to exist in more than one form or crystal structure. It describes the ability of a substance to exist as two or more crystalline phases that have different arrangements of the molecules in the solid-state but are otherwise identical in terms of chemical content. Polymorphism can potentially be found in any crystalline material including polymers, minerals, and metals, and is related to allotropy, which refers to chemical elements. This can have significant differences in their physical properties even though they are chemically identical. The complete morphology of a material is described by polymorphism and other variables such as crystal habit, amorphous fraction, or crystallographic defects.
Polymorphism is relevant to the fields of pharmaceuticals, agrochemicals, pigments, dyestuffs, foods, and explosives. It affects many different types of compounds ranging from mineralogy, metallurgy, materials science, food industry to the pharmaceutical industry.
Polymorphism is the ability of solid materials to exist in two or more crystalline forms with different arrangements or conformations of the constituents in the crystal lattice. It is the ability of a solid substance to crystallize into more than one different crystal structure. When polymorphism exists as a result of a difference in crystal packing, it is called packing polymorphism. They may interconvert depending on conditions. Usually, the metastable kinetic form converts to the stable thermodynamic form.
Polymorphism can also result from the existence of different conformers of the same molecule in conformational polymorphism. In pseudopolymorphism the different crystal types are the result of hydration or solvation. This is more correctly referred to as solvomorphism as different solvates have different chemical formulae. An example of an organic polymorph is glycine, which is able to form monoclinic and hexagonal crystals. Silica is known to form many polymorphs, the most important of which are; α-quartz, β-quartz, tridymite, cristobalite, moganite, coesite, and stishovite. A classical example is the pair of minerals, calcite, and aragonite, both forms of calcium carbonate.
Types of Polymorphism
It can be classified into two types according to their stability with respect to the different range of temperature and pressure.
- Monotropic System – Only one polymorph is stable at all reasonable temperatures. e.g. metolazone
- Enantiotropic system – One polymorph is stable over one temperature range, another polymorph is stable over a different temperature range – e.g. carbamazepine and acetazolamide.
The term polymorphism should not be confused with the notion of solvates, which are crystalline forms containing solvent molecules as well as the main compound(s). An analogous phenomenon for amorphous materials is polyamorphism when a substance can take on several different amorphous modifications. This phenomenon was discovered in the early nineteenth century but only since the middle of the twentieth century have its wider implications been appreciated by scientists investigating the crystallization, properties, and interconversion of solid phases.