At typical atmospheric pressures and temperatures, liquid helium is a physical state of helium. It’s used to generate low temperatures. Superfluidity may be observed in liquid helium. The inert, nonflammable gas is employed in balloons as well as scientific research (e.g., meteorological). It is also used in arc welding inert gas shielding, filling light bulbs, as a carrier gas in chromatography, and as a substitute for nitrogen in deep diving air supplies.
Only at the extraordinarily low temperature of 269 °C (452.20 °F; 4.15 K) does the chemical element helium exist in liquid form at standard pressure. Its boiling point and critical point are determined by whether it contains the common isotope helium-4 or the rare isotope helium-3. These are the only two stable isotopes of helium.
Liquefaction
At very low temperatures, quantum mechanical phenomena allow the emergence of a macroscopic “superfluid” state with optimal, classical fluid dynamic behavior in liquid helium. A superfluid is a condition of matter in which it behaves like a non-viscous fluid. The substance behaves differently from any other liquid in that it glides over surfaces without friction, circulating within any container, subject only to its own inertia, and passing through any and all impediments or pores.
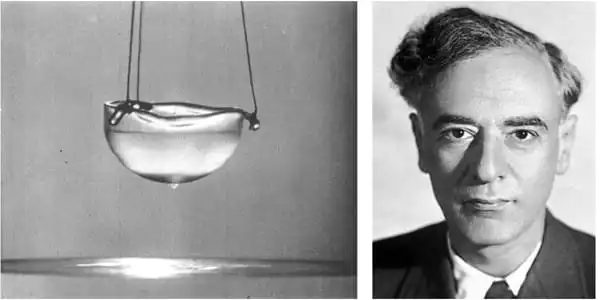
Helium was first liquefied on July 10, 1908, at the University of Leiden in the Netherlands by Dutch physicist Heike Kamerlingh Onnes. Helium-3 was unknown at the time since the mass spectrometer had not yet been invented. Liquid helium has been used as a cryogenic refrigerant (which is used in cryocoolers) in recent decades, and liquid helium is produced commercially for use in superconducting magnets such as those used in magnetic resonance imaging (MRI), nuclear magnetic resonance (NMR), Magnetoencephalography (MEG), and physics experiments such as low-temperature Mössbauer spectroscopy.
Characteristics
Because of the minimal affinity between the helium atoms, the temperature required to make liquid helium is low. Because helium is a noble gas, the interatomic interactions are already weak, but the effects of quantum mechanics diminish the interatomic attractions even further. Helium has a low atomic mass of roughly four atomic mass units, hence these are significant. Liquid helium has lower zero-point energy if its atoms are less constrained by their neighbors. As a result, the ground state energy of liquid helium might drop due to a naturally occurring increase in its average interatomic distance. However, the effects of helium’s interatomic interactions are significantly weaker at longer distances.
Because of the element’s extremely weak interatomic interactions, helium remains a liquid at atmospheric pressure all the way from its liquefaction point to absolute zero. Only at extremely low temperatures and high pressures does liquid helium solidify. [clarification required] Both helium-4 and helium-3 experience superfluid transitions at temperatures below their liquefaction thresholds.
Liquid helium-4 and the extremely rare liquid helium-3 are not totally miscible. A mixture of the two isotopic phases separates below 0.9 kelvin at their saturated vapor pressure into a normal fluid (mainly helium-3) that floats on a denser superfluid (primarily helium-4). This phase separation occurs because separating reduces the overall mass of liquid helium’s thermodynamic enthalpy.