The mercury cycle is a biogeochemical cycle that is influenced by both natural and anthropogenic processes that transform mercury into various chemical forms and environments. Mercury can be found in the Earth’s crust and in various forms on its surface. It may be elemental, inorganic, or organic in nature. There are three oxidation states of mercury: 0 (elemental mercury), I (mercurous mercury), and II (mercuric mercury).
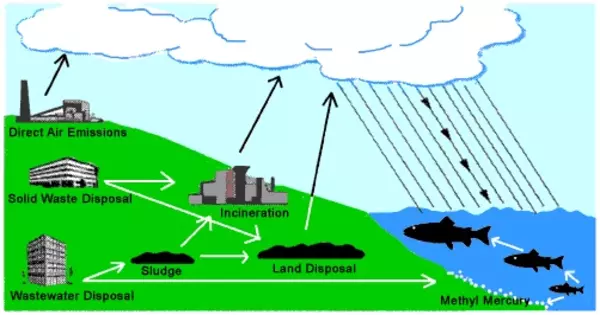
Mercury eventually settles back to the Earth’s surface via deposition, which can be dry or wet (i.e., mercury falling back to the ground via rain or snow). Plants and other organisms can absorb mercury. Certain microorganisms in aquatic environments can convert elemental mercury into methylmercury, a highly toxic form that bioaccumulates in the food chain.
Natural and anthropogenic sources emit gaseous elemental mercury (Hg(0)) into the atmosphere. It can linger in the atmosphere for extended periods of time and travel long distances. Mercury can be returned to the Earth’s surface via both dry and wet deposition. It can be washed out of the atmosphere by rainfall or snowfall in wet deposition.
Mercury emissions to the atmosphere can come from either primary sources (releasing mercury from the lithosphere) or secondary sources (exchanging mercury between surface reservoirs). Over 5000 metric tonnes of mercury are released into the atmosphere each year as a result of primary emissions and secondary re-emissions.
Mercury is a naturally occurring element, but human activities such as the use of fossil fuels and mining have increased its release into the environment significantly. Mercury can cycle through the atmosphere, soil, bodies of water, and living organisms, resulting in bioaccumulation and biomagnification in the food chain. Because mercury is a toxic substance, it can be harmful to both wildlife and humans.
The concentration of methylmercury increases due to biomagnification as smaller organisms containing methylmercury are consumed by larger organisms. Human activities, particularly industrial processes such as coal combustion and gold mining, have increased mercury emissions into the atmosphere significantly. This anthropogenic input has disrupted the natural mercury cycle, resulting in higher mercury levels in the environment.
Because of the environmental and public health risks associated with mercury contamination, efforts are being made to study and manage the mercury cycle. Controlling mercury emissions, particularly those caused by human activity, is critical for reducing the impact on ecosystems and human health.