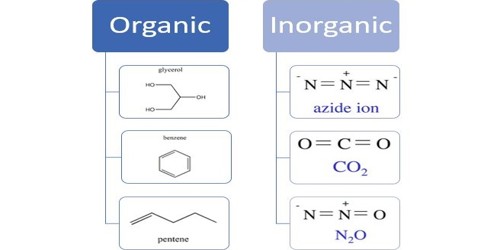
An inorganic compound is a chemical compound that is not an organic compound. That means it is not a carbon-based compound. Inorganics include salts, metals, substances made from single elements and any other compounds that don’t contain carbon bonded to hydrogen. The example for inorganic compounds includes non-metals, salts, metals, acids, bases, substances which are made from single elements. They can be considered as a compound that does not contain a carbon-to-hydrogen bond, also called a C-H bond. So, it is a compound that does not contain hydrocarbon groups.
Inorganic compounds comprise most of the Earth’s crust, although the compositions of the deep mantle remain active areas of investigation. Example: sodium chloride, NaCl, carbon dioxide, CO2, diamond (pure carbon), silver, sulfur, etc. They may consist of heavy metals and toxic elements (e.g., lead, mercury, chromium, arsenic, etc.) in pure form or combined with other elements. Examples include carbon monoxide, carbon dioxide, carbonates, carbides, cyanides, cyanates, and thiocyanates.
Types of inorganic compounds
The division between the two types of a compound is not absolute. Since many inorganic compounds contain some type of metal (alkali, alkaline, transition, etc.), they tend to be able to conduct electricity. Some carbon-containing compounds are traditionally considered inorganic. Many chemicals in nature are not compounds but are ions. They are often quite simple, as they do not form the complex molecular bonds that carbon makes possible. Sodium, chloride, and phosphate ions are essential for life, as are some inorganic molecules such as nitrogen, carbon dioxide, water, and oxygen. Aside from these simple ions and molecules, virtually all compounds covered by biochemistry contain carbon and can be considered organic or organometallic.
Major types of inorganic compounds may include:
- Minerals, such as salt, asbestos, silicates, …
- Alloys, like brass, bronze, …
- Most compounds involving non-metallic elements, like silicon, phosphorus, chlorine, oxygen, for example, water.