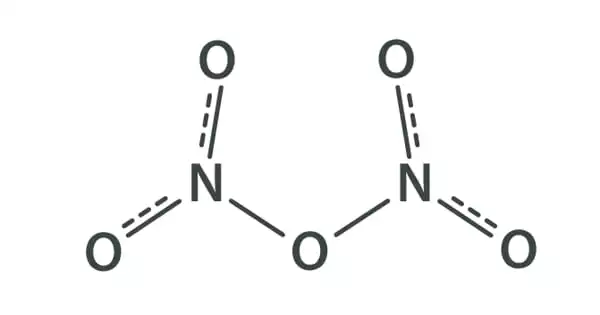
Dinitrogen pentoxide, commonly known as nitrogen pentoxide or nitric anhydride, is a chemical molecule having the formula N2O5. It is a kind of nitrogen oxide. It is a binary nitrogen oxide, which is a type of substance that simply contains nitrogen and oxygen. The anhydride form of nitric acid can exist as a colorless crystal. It exists in the form of colorless crystals that melt at 41 °C. It has a boiling point of 47 degrees Celsius and sublimes slightly above room temperature, producing a colorless gas.
Dinitrogen pentoxide is an unstable and potentially dangerous oxidizer that once was used as a reagent when dissolved in chloroform for nitrations but has largely been superseded by nitronium tetrafluoroborate (NO2BF4). It is unstable compound decompose spontaneously at room temperature into nitrogen dioxide and oxygen. N2O5 is a rare example of a compound that adopts two structures depending on the conditions. The solid is a salt, nitronium nitrate, consisting of separate nitronium cations [NO2]+ and nitrate anions [NO3]–; but in the gas phase and under some other conditions it is a covalently-bound molecule.
Properties
Dinitrogen Pentoxide is a white crystal form of the anhydride form of nitric acid. Because it is a relatively unstable chemical, it decomposes spontaneously at room temperature. It produces a high concentration of nitronium cation when combined with nitric acid. Dinitrogen Pentoxide is a chemical that is unstable and somewhat explosive, however it has no commercial value at the moment.
- Density: 1.64 g/cm³
- Molecular Weight/ Molar Mass: 108.01 g/mol
- Boiling Point: 47 °C
- Melting Point: 41 °C
- Odour: No odour
- Appearance: White solid
- Solubility: Soluble in alcohol and ether
Preparation
A recommended laboratory synthesis entails dehydrating nitric acid (HNO3) with phosphorus(V) oxide:
P4O10 + 12 HNO3 → 4 H3PO4 + 6 N2O5
Another laboratory process is the reaction of lithium nitrate LiNO3 and bromine pentafluoride BrF5, in the ratio exceeding 3:1. The reaction first forms nitryl fluoride FNO2 that reacts further with the lithium nitrate:
BrF5 + 3 LiNO3 → 3 LiF + BrONO2 + O2 + 2 FNO2
FNO2 + LiNO3 → LiF + N2O5
The compound can also be created in the gas phase by reacting nitrogen dioxide NO2 or N2O4 with ozone:
2 NO2 + O3 → N2O5 + O2
However, the product catalyzes the rapid decomposition of ozone:
2 O3 + N2O5 → 3 O2 + N2O5
Dinitrogen pentoxide is also formed when a mixture of oxygen and nitrogen is passed through an electric discharge. Another route is the reactions of Phosphoryl chloride POCl3 or nitryl chloride NO2Cl with silver nitrate AgNO3.
Uses
As a nitrating agent, dinitrogen pentoxide was utilized. However, because of its unstable nature, Nitronium Tetrafluoroborate is now utilized instead of N2O5. Making TNT from N2O5 is a potentially bad use of it.
It is utilized in high-fuel rockets as a strong oxidizer. It is utilized in solvents that are not dependent on water so that water-sensitive compounds can be easily nitrated. It is also used to denote the light intensities transmitted when the cell was occupied by disintegrating nitrogen pentoxide and when the cell was occupied by entirely decomposed nitrogen pentoxide.