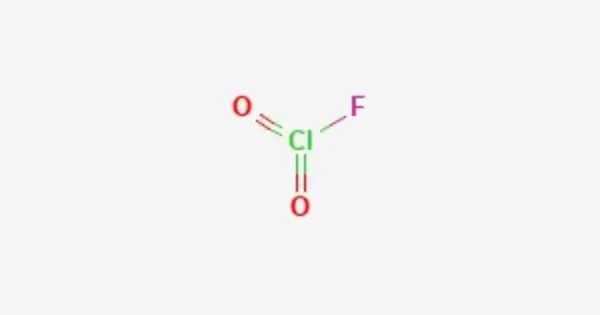
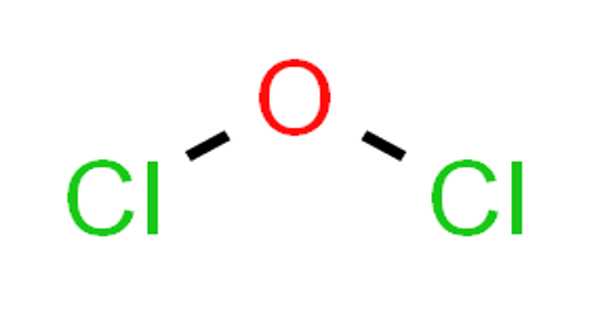
Chlorosyl fluoride is an inorganic compound of chlorine, fluorine, and oxygen with the chemical formula F−Cl=O. It is a rare, highly reactive interhalogen compound with the chemical formula ClOF. It is a colorless gas at room temperature, though it is seldom encountered due to its instability and extreme reactivity. Structurally, ClOF features a chlorine atom bonded to an oxygen atom, which is also bonded to a fluorine atom, forming a bent molecule with a T-shaped geometry due to lone pairs on chlorine.
ClOF has no significant practical applications due to its instability but is of interest in theoretical chemistry and fluorine chemistry research. It is toxic, corrosive, and requires specialized handling in inert environments like Teflon or metal containers. Spectroscopic studies reveal its molecular properties, but its fleeting existence limits detailed exploration. Extreme caution is needed due to its potential to form hazardous byproducts upon decomposition.
Properties
- Chemical formula: FClO
- Molar mass: 70.45 g·mol−1
Synthesis
Synthesized typically by reacting chlorine monofluoride (ClF) with oxygen sources under controlled conditions, ClOF is challenging to isolate because it decomposes readily, often reacting with moisture, glass, or organic materials. Its high reactivity stems from the weak Cl-O bond and the electronegative nature of fluorine and oxygen, making it a potent oxidizer.
- Partial hydrolysis of chlorine trifluoride in diluted gas phase at low temperatures.
- Reaction of chlorine trifluoride with nitric acid.
Chemical properties
Chlorosyl fluoride is thermolabile and disproportionates to ClF and FClO2:
2 FClO → ClF + FClO2
2 FClO → 2 ClF + O2
Synthesis
Preparation Methods:
Partial Hydrolysis of Chlorine Trifluoride (ClF₃): FClO is primarily synthesized through the controlled hydrolysis of ClF₃ with water (H₂O) under slow-flow, low-temperature conditions, yielding FClO as the main product.sciencedirect.comntrs.
Reaction with Nitric Acid: Another method involves reacting ClF₃ with nitric acid.
Challenges: Due to its high reactivity, FClO is typically prepared in situ and studied immediately, as it is difficult to store.
Occurrences
Natural Occurrence: There is no evidence that chlorosyl fluoride occurs naturally in the environment. It is a synthetic compound primarily studied in laboratory settings.
Proposed Atmospheric Relevance: FClO has been suggested as a potential intermediate in atmospheric reactions involving chlorine fluorides (e.g., ClF, Cl₂O, ClF₃O). However, its high reactivity limits its persistence in the atmosphere.
Applications
FClO is not widely used industrially due to its instability and reactivity. It is mainly of interest in fundamental and theoretical chemistry for studying hypervalent compounds and testing quantum-chemical models.
It is produced as a byproduct or intermediate in reactions involving chlorine trifluoride, particularly in processes related to the semiconductor industry or nuclear fuel processing where ClF₃ is used.
Safety and Handling
- Reactivity Hazards: FClO is extremely reactive, similar to its precursor ClF₃, which is known to ignite or react explosively with many organic and inorganic materials, including water, wood, and asbestos.
- Storage: Like ClF₃, FClO can be handled in certain metals (e.g., steel, copper, aluminum) due to the formation of a protective metal fluoride layer, but this layer can fail under certain conditions, leading to violent reactions.en.